Abstract
Thousands of people around the world are waiting for life-saving organ transplants, but there are not enough organs to meet the demand. Gene-edited organs from animals, especially pigs, could help solve the organ shortage. Using a technology called gene editing, scientists can change the DNA of pig organs, making them safer and more compatible with human bodies by turning off genes that cause the body to attack the foreign organ and removing dangerous virus genes. This technology is already being tested—pig kidneys and hearts have been transplanted into patients, providing important information that will help improve future procedures. Scientists are also studying how transplanted pig cells could treat diseases like diabetes and Parkinson’s. While challenges and ethical questions remain, this technology has the potential to save thousands of lives every year. With more research, gene-edited organs could give people in need of transplants a second chance at life.
The World Needs More Organs for Transplants
Imagine being really sick and needing a new heart or kidney to survive, but being told you might have to wait years—if an organ becomes available at all. This is the reality for thousands of people who need organ transplants. While these life-saving procedures have helped millions, the demand for organs far exceeds the supply. Behind every transplant is a person—often someone very sick, who may need machines to keep them alive while they wait. For many families, each day without an available organ is a race against time.
Organ transplants rely on organ donors—people who provide an organ, either after they pass away or, in some cases, while they are still alive, such as donating one kidney or part of the liver. The person receiving the transplant is called the recipient. Commonly transplanted organs include kidneys, hearts, livers, and lungs, but the waiting lists for these organs are long. In the United States, over 100,000 people are waiting for an organ transplant, but only about 30,000 transplants will happen this year. Some patients may wait years for an organ, and sadly, many do not survive that long.
Even when a matching organ becomes available, there is another big challenge: rejection. The recipient’s immune system, which protects the body from dangerous invaders, may see the transplanted organ as something unfamiliar and attack it, like it would a virus or bacteria. To prevent this, patients take medications to weaken their immune systems, but these drugs have side effects and are not always effective at stopping rejection.
To address the shortage of organs, scientists are exploring a surprising source: animals—specifically pigs (Figure 1). A new field called xenotransplantation is studying how organs from animals could be used to save human lives. But if the human body can reject an organ from another person, how could it possibly accept one from a completely different species?
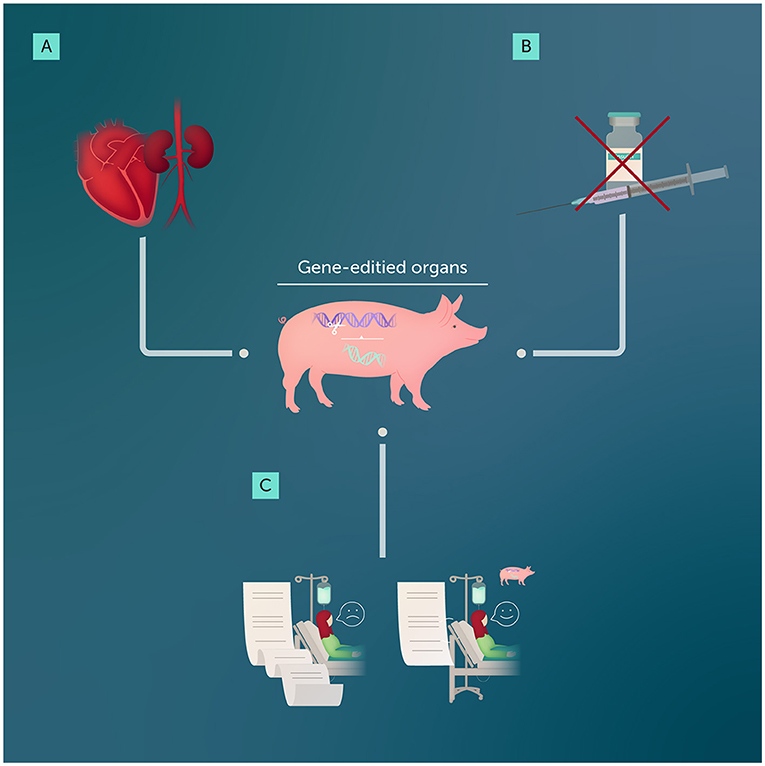
- Figure 1 - Gene editing can help to make organs from other animals suitable for transplanting into humans.
- (A) Early cases in which hearts and kidneys from pigs were transplanted into humans have provided scientists and doctors with the valuable information they need to make xenotransplantation safer and less likely to result in organ rejection. (B) Gene-edited pigs could provide pancreas cells that could reduce the need for insulin injections in people with diabetes. (C) Overall, successful xenotransplantation using gene-edited organs could shorten the wait list for donor organs, saving thousands of lives every year.
Emerging Technology: Gene-Edited Organs
To make animal organs suitable for human transplants, scientists are using a powerful tool called gene editing. Gene editing allows scientists to make precise changes to an animal’s DNA—the instructions that tell cells how to grow and function. One of the most commonly used tools for gene editing is called CRISPR-Cas9. You can generally think of this technique like a pair of molecular scissors that can cut and paste specific bits of DNA exactly where they are needed.
In xenotransplantation, gene editing addresses two big problems. First, the human immune system naturally sees a pig organ as a threat and attacks it, causing rejection. To prevent this, scientists edit the DNA of pigs while the animals are still embryos, long before they are born. These changes include “turning off” certain pig genes that trigger the human immune system and adding human genes to the pig’s DNA, to make the organ more compatible with the human body. The pig organs grow with these edits already in place, and when they are big enough, they are ready for transplant. The second problem gene editing addresses is safety. Like humans and many other animals, pigs have leftover virus DNA inside their own DNA—kind of like old “genetic scars” from infections their ancestors had long ago. These virus bits usually do not do anything, but there is a small chance they could become active and cause problems in humans after a transplant. Using gene editing, scientists can remove these virus genes to help make the organs safer for people who receive them.
With gene editing, scientists can engineer pig hearts or kidneys to work inside human bodies without causing harm (Figure 1). In some cases, scientists need to make multiple edits to the pig’s DNA—usually around 10, but sometimes up to 69—to ensure the organs are safe and effective for transplantation [1].
Tech to the Rescue
Gene-edited organs are already showing promise for saving lives. In 2024, doctors successfully transplanted a kidney from a pig into a human patient [2]. This groundbreaking procedure showed that xenotransplantation could work and paved the way for future advancements. The patient lived for 2 months after the surgery, and although he passed away, his death was not related to the transplant. Gene-edited pig hearts have also been transplanted into human patients [3, 4]. While these patients did not survive long-term, these early cases provide valuable information to improve the technology and make it safer.
Gene-edited pig organs could also help treat diseases like diabetes. Diabetes occurs when the pancreas, an organ that produces insulin, stops working properly and does not produce enough insulin. Insulin is a hormone that helps move sugar from the blood into the body’s cells, where it is used for energy. People with type 1 diabetes often take daily insulin injections to manage their blood sugar. Scientists are exploring the idea of transplanting just the insulin-producing cells, called islet cells, from the pancreas of gene-edited pigs into patients with diabetes (Figure 1). These cells might restore the function of the pancreas, and reduce or even eliminate the need for daily injections.
Beyond diabetes, gene-edited pigs could provide cells to treat other conditions. Parkinson’s disease is a brain condition that affects movement and coordination. Parkinson’s patients might be treated with transplanted pig brain cells, which could replace damaged cells in the human brain, improving a patient’s symptoms and quality of life. Scientists are also studying how cells from gene-edited pigs might repair other damaged tissues or treat diseases like liver failure.
Big Challenges, Bigger Opportunities
Although much work remains, the use of gene-edited organs in xenotransplantation is a promising step toward solving the organ shortage crisis. One remaining hurdle is the risk of rejection. Even with gene editing, doctors must carefully monitor patients and use special medicines to help their bodies accept the transplants. Scientists are working to improve the gene-editing process to make pig organs even more compatible with human bodies.
Safety is another concern. As we mentioned, pigs naturally carry viruses in their DNA that might harm humans if transmitted during a transplant. Removing these virus genes with gene-editing tools lowers this risk, but more testing is needed to make sure the transplants are completely safe.
There are also ethical questions. For example, how do we balance the need to save human lives with ensuring the welfare of animals used for transplants? These important conversations will shape how xenotransplantation is developed and used in the future.
With more research and advancements in gene editing to overcome these challenges, xenotransplantation could eventually save thousands of lives each year. Imagine a world where no one has to wait years for a life-saving organ or worry about their body rejecting a transplant. Scientists, doctors, and policymakers are working together to make this vision a reality, offering hope to patients and families around the world.
Glossary
Organ Transplants: ↑ A medical procedure where a healthy organ from one person or animal is placed into another person to replace a damaged or failing organ.
Donor: ↑ A person or animal who gives an organ for a transplant, either after they pass away or, in some cases, while they are still alive.
Recipient: ↑ The person who receives an organ during a transplant.
Rejection: ↑ When the recipient’s immune system attacks and damages a transplanted organ, treating it as a threat.
Immune System: ↑ The body’s defense system that fights off germs and infections. It can sometimes attack a transplanted organ, thinking it is a dangerous invader.
Xenotransplantation: ↑ Transplanting organs or cells from one species (like pigs) into another species (like humans).
Gene Editing: ↑ A process that allows scientists to change DNA—the instructions for how cells work—to make animals or plants better suited for specific purposes, like creating organs compatible with humans.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Co-written and edited by Susan Debad Ph.D., graduate of the UMass Chan Medical School Morningside Graduate School of Biomedical Sciences (USA) and scientific writer/editor at SJD Consulting, LLC. Figure created by Somersault18:24.
Original Source Article
↑Cooper, D. K., Cozzi, E., Ling, G. and Meyerson, B. 2024. “Genomics for transplants. Gene-editing organs for transplantation advancements”, in Top 10 Emerging Technologies of 2024 Flagship Report. World Economic Forum. Available at: https://www.weforum.org/publications/top-10-emerging-technologies-2024/
References
[1] ↑ Anand, R. P., Layer, J. V., Heja, D., Hirose, T., Lassiter, G., Firl, D. J., et al. 2023. Design and testing of a humanized porcine donor for xenotransplantation. Nature 622:393–401. doi: 10.1038/s41586-023-06594-4
[2] ↑ Mallapaty, S., and Kozlov, M. 2024. First pig kidney transplant in a person: what it means for the future. Nature 628:13–4. doi: 10.1038/d41586-024-00879-y
[3] ↑ Griffith, B. P., Goerlich, C. E., Singh, A. K., Rothblatt, M., Lau, C. L., Shah, A., et al. 2022. Genetically-modified porcine-to-human cardiac xenotransplantation. N. Engl. J. Med. 387:35–44. doi: 10.1056/NEJMoa2201422
[4] ↑ Cooper, D. K. C., and Cozzi, E. 2024. Clinical pig heart xenotransplantation - where do we go from here? Transpl. Int. 37:12592. doi: 10.3389/ti.2024.12592