Abstract
Your body has an organ called the pancreas, which contains cells that do a very important job: they make insulin, a hormone that helps control the amount of sugar in your blood. Insulin acts like a key, unlocking cells so they can use sugar for energy. In type 1 diabetes, the body’s defense system mistakenly attacks these insulin-producing cells. Without insulin, sugar builds up in the blood, which can be harmful. To stay healthy, kids and teens with diabetes need to take insulin every day, through injections or pumps. But insulin does not fix the problem. Scientists are working on an exciting new idea: growing tiny “mini-organs” in the lab that act like the cells in the pancreas and make insulin to control blood sugar. The plan is to transplant these mini-organs into people with diabetes, so their bodies can make insulin again. This research could help kids with diabetes live healthier, easier lives.
What is Diabetes?
Did you know your body contains tiny “islands” that help keep your energy balanced? These islands, called islets of Langerhans, are found in your pancreas, an organ in your belly. They are called islets (which means “little islands”) because under a microscope they look like tiny islands made up of different types of cells. One very important type of cell in these islets is called the beta cell. Beta cells have an important job: they produce insulin, a hormone that acts like a key, unlocking your cells so that a type of sugar called glucose can move into them from your blood to be used as fuel, especially to make your brain sharp and muscles strong (Figure 1A).
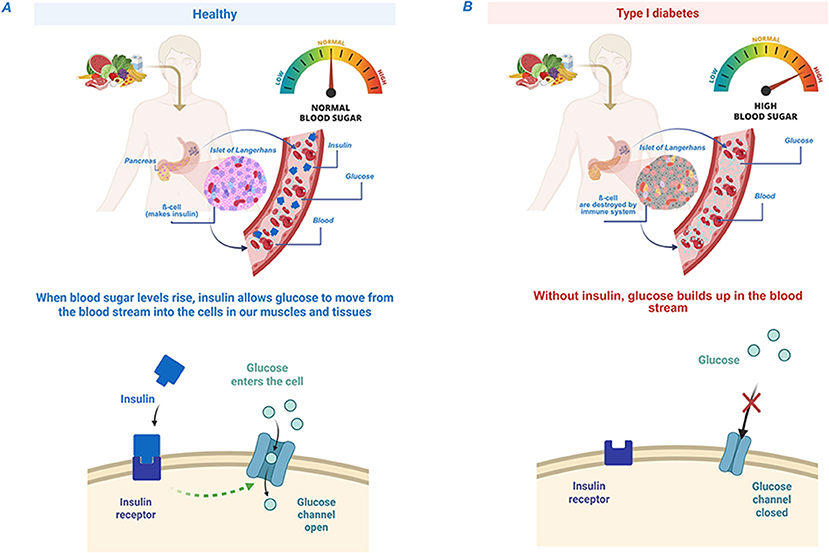
- Figure 1 - (A) In healthy people, insulin, a hormone that acts like a key, unlocks your cells so glucose from the blood can enter and give you energy.
- (B) In people with type I diabetes, the body can’t make insulin, so the “key” is missing—glucose stays trapped in the blood and can’t get into the cells [Created in BioRender. Berishvili, E. (2025) https://BioRender.com/j06z479].
In people with a condition called type 1 diabetes, the body’s defense system—the immune system—mistakenly attacks and destroys insulin- making beta cells in the islets [1]. More than 1.5 million kids and teens worldwide suffer from this condition. Without insulin, the sugar in the blood cannot move inside the cells. Instead, it builds up in the blood, causing dangerously high blood sugar levels, which can lead to serious health problems (Figure 1B; to learn more about diabetes in kids, see this Frontiers for Young Minds article.)
Why is High Blood Sugar Bad?
Blood sugar may sound harmless—it is just sugar, right? But when there is too much of it, it can harm the body. Imagine pouring too much syrup on pancakes. The syrup spills over, makes everything sticky, and becomes hard to clean up. In your body, too much sugar in the blood works similarly. It can damage vital organs like the eyes, kidneys, and heart, and it can even harm the nerves. High blood sugar can also make it harder for cuts and wounds to heal.
For kids and teens with type 1 diabetes, managing blood sugar becomes a daily job. They need to check their blood sugar levels and take insulin shots to keep it within a safe range. Although insulin helps to keep blood sugar at safe levels, it is not a cure (Figure 2).
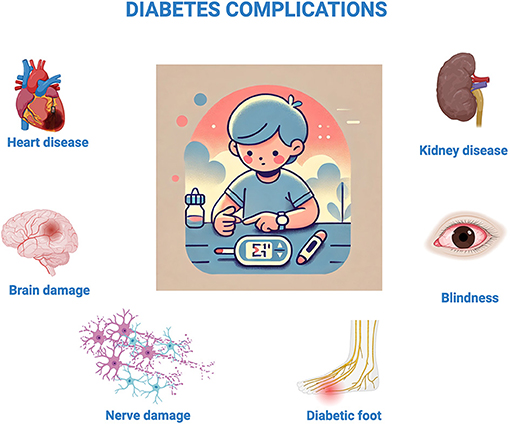
- Figure 2 - When blood sugar levels are too high for too long, they can cause problems (called “complications”) for various organs or tissues—including the heart, kidneys, brain, eyes, skin, and nerves [Created in BioRender.
- Berishvili, E. (2025) https://BioRender.com/r40v661].
Why is Insulin not Enough?
Insulin injections or pumps are lifesaving for people with diabetes, but they do not solve all the problems. People with diabetes must constantly monitor their blood sugar, especially when they eat, exercise, or sleep. This can be tiring, and despite their efforts, blood sugar levels can still go too high or too low.
When blood sugar drops too low, it is called hypoglycemia, which can make a person feel dizzy, shaky, or weak. If untreated, hypoglycemia can become dangerous. On the other hand, high blood sugar can make a person feel thirsty or tired and cause long-term health issues, as we described. This is why scientists are searching for a way to help people manage their blood sugar naturally, without the need for constant insulin shots.
Creating Mini-Organs: A New Idea
The future of diabetes treatment is full of promise. Scientists are working on an exciting solution: creating mini-organs—tiny organ-like structures grown from cells in a lab—which are called islet organoids. These clusters of insulin-producing cells mimic the real islets in the pancreas, naturally helping control blood sugar levels. Mini-organs could be life-changing for people with diabetes, especially kids and teens. That’s because growing up with diabetes can make it extra hard to keep blood sugar levels steady, since their bodies are always changing. By transplanting these tiny insulin-producing factories into the body, people with diabetes could live more normal lives. Inside the body, they would work just like normal islets, producing insulin whenever blood sugar levels get too high. No more insulin shots, no more constant monitoring—just a body that knows how to control its own blood sugar.
But creating mini-organs is not easy. They need to be the right size, contain the right types of cells, and work properly once inside the body. Scientists are working hard to figure out the best way to build and transplant mini-organs, so they work just as well as natural islets. They also need to make sure the mini-organs do not get “exhausted” from working too hard. If someone’s blood sugar is very high, these new islets could get tired from producing so much insulin (imagine how tired you feel if you run for too long). To prevent this, scientists plan to transplant enough mini-organs and add helper cells to support them. This way, each mini-organ only handles part of the work, so they do not get overworked or burned out.
How do We Make Mini-Organs?
Creating mini-organs is a careful and precise process. Scientists begin by taking cells from a pancreas and growing them in a lab. The cells are combined into clusters that mimic real islets, ensuring they can produce insulin and respond to changes in blood sugar levels. Scientists also add other cells to support the islet cells and help them survive and function better inside the body. These extra cells act like maintenance workers, keeping everything running smoothly. Once the mini-organs are ready, they are tested to make sure they can produce insulin when needed [2].
One big challenge with mini-organs is making sure the body does not attack them. Remember how the immune system mistakenly attacks the islets in people with type 1 diabetes? The same thing could happen to the mini-organs if we are not careful. To prevent this, scientists are working on ways to protect them. One idea is to cover the mini-organs with special cells or materials that hide them from the immune system, like putting on an invisible shield. This would allow them to do their job without being destroyed. You might be wondering: will this invisible shield keep protecting the mini-organs over time? Scientists are testing different materials to make sure the shield is strong and long-lasting inside the body. This invisible shield would be made of materials that can safely stay inside the body for a long time, so the mini-organs remain protected for many years—maybe even for a person’s whole life—keeping the insulin-producing cells safe and working.
Why not just put the same protective shield around the islets that are already in the pancreas? Well, it is very hard to do that inside the body. The islets in the pancreas are tiny and spread out, so doctors cannot easily coat each one without taking them out. Also, by the time someone has type 1 diabetes, most of their islet cells have already been destroyed. So, we need to make new healthy islet cells (as mini-organs) and protect those. Once they are sufficiently protected, the mini-organs can be transplanted into the body, where they take over the job of controlling blood sugar (Figure 3).
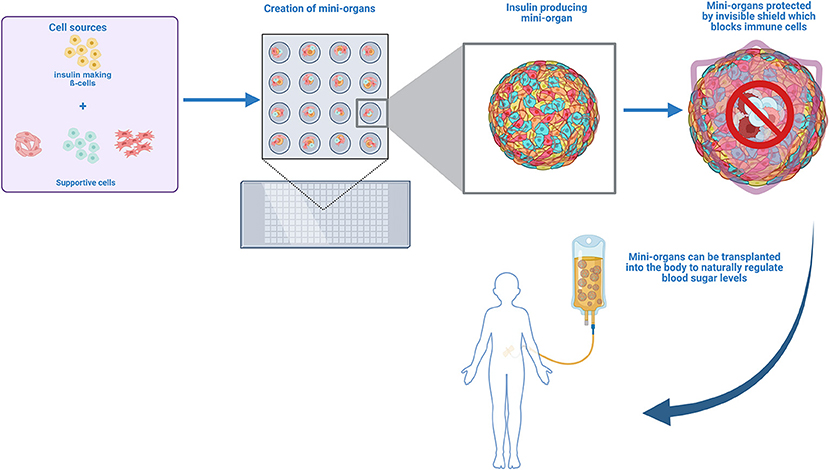
- Figure 3 - Insulin-making cells and supportive cells are placed on special micro-dishes to grow mini-organs that are all same size and have similar amounts of each cell type.
- Scientists are exploring ways to protect these mini-organs from the immune system by developing an invisible shield, which will help them to function in the body without being attacked. In the future, these protected mini-organs could be transplanted into people with diabetes to help naturally regulate blood sugar levels [Created in BioRender. Berishvili, E. (2025) https://BioRender.com/v32s636].
The Future of Diabetes Treatment
The journey to the creation of mini-organs is still ongoing, but every discovery brings us closer to changing lives [3]. How close are we to testing these mini-organs in people? So far, scientists have tried them in the lab and in animals, but testing in humans is the next big step. Before doctors can put these mini-organs into patients, they must be very sure that it is safe and that it works well. If all goes well, the first tests in patients could happen in the coming years, but it is hard to know exactly when. In the meantime, scientists are refining methods to grow and protect these organoids, ensuring they work seamlessly inside the body. In the future, scientists hope to make mini-organs using special cells called stem cells (see here for more info) or the person’s own cells, making them invisible to the immune system and thus keeping them safe from attack.
Right now, managing diabetes can feel overwhelming. But with the development of mini-organs, there is hope that one day, diabetes could be a thing of the past. Soon, mini-organs could replace the need for insulin pumps and injections entirely. Imagine a world where diabetes is no longer a life-defining condition—a world where children with diabetes can focus on their dreams rather than their disease. This could mean a future where kids with diabetes can focus on being kids—playing, learning, and enjoying life—without the burden of insulin shots and blood sugar monitoring.
It is an exciting time in diabetes research, and with each new discovery, we move one step closer to a cure. Another important question is whether this future treatment will be affordable for children everywhere, even in developing countries. New medical breakthroughs can be expensive at first, so scientists and doctors are thinking about how to lower the costs. As they get better at making mini-organs and more people start using them, the treatment should become less expensive. The goal is that kids with diabetes all around the world can benefit from mini-organs, not just those in wealthy countries. After all, a cure is most exciting when it can reach everyone who needs it. Maybe one day, no one will have to worry about diabetes anymore—that is the amazing future we are working toward.
Glossary
Islets of Langerhans: ↑ Tiny clusters of cells scattered in the pancreas. They make insulin and help keep blood sugar levels normal.
Pancreas: ↑ An organ in the belly that helps with digestion and makes insulin to control blood sugar.
Beta Cell: ↑ Tiny cell inside the islets of the Lagerhans. They make insulin to help control blood sugar.
Insulin: ↑ A hormone made by the pancreas that acts like a key, helping sugar move from the blood into the cells to give them the energy they need to perform their functions.
Type 1 Diabetes: ↑ A type of diabetes in which the immune system attacks the insulin-making cells in the pancreas, so the body cannot make insulin.
Immune System: ↑ The body’s defense system, which protects the body from germs and illnesses.
Hypoglycemia: ↑ A condition in which blood sugar levels are too low.
Organoids: ↑ Tiny groups of cells made in a lab that work like the actual organ. Islet organoids can produce insulin and help control blood sugar.
Stem Cells: ↑ Cell that can form all different cell types present in the body.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by grants from the European Commission (Horizon 2020 Framework Program; VANGUARD grant 874700), the Breakthrough T1D International Foundation ( grants 3-SRA-2020-926-S-B and 3-SRA-2023-1441-S-B) and the Swiss National Science Foundation (grant 310030_173138).
AI Tool Statement
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Original Source Article
↑Wassmer, C. H., Lebreton, F., Bellofatto, K., Perez, L., Cottet-Dumoulin, D., Andres, A., et al. 2022 Bio-engineering of pre-vascularized islet organoids for the treatment of type 1 diabetes. Transpl. Int. 35:10214. doi: 10.3389/ti.2021.10214
References
[1] ↑ Fonseca, L. M., Krause, N., Lebreton, F., and Berishvili, E. 2025. Recreating the endocrine niche: advances in bioengineering the pancreas. Artif. Organs. 49:541–55. doi: 10.1111/aor.14950
[2] ↑ Wassmer, C. H., Lebreton, F., Bellofatto, K., Perez, L., Cottet-Dumoulin, D., Andres, A., et al. 2022 Bio-engineering of pre-vascularized islet organoids for the treatment of type 1 diabetes. Transpl. Int. 35:10214. doi: 10.3389/ti.2021.10214
[3] ↑ Hering, B. J., Rickels, M. R., Bellin, M. D., Millman, J. R., Tomei, A. A., García, A. J., et al. 2025. Advances in cell replacement therapies for diabetes. Diabetes 74:1068–77. doi: 10.2337/db25-0037
