Abstract
People usually take medicines by swallowing a tablet or pill, but many important medicines cannot be taken by mouth. Vaccines, for example, must be injected using a needle and syringe. You might know people who have diabetes, a condition in which the body does not make enough of the hormone insulin. Insulin allows your cells to use the sugar in your blood for energy. People with diabetes must take insulin to be able to lower their blood sugar levels. Unfortunately, people cannot take insulin by mouth because the acid in the stomach will attack it like food. Insulin is also large and cannot cross the wall of the intestine to get into the blood, which is where it needs to go. Instead, people must take insulin by injecting it into the blood. But what if medicines, like insulin, could be designed so they could be swallowed instead of being injected?
The Problem with Peptides
We all hate injections! In fact, about one person in 20 is really afraid of needles. Sometimes there is no choice because not all medicines can be swallowed as a pill or tablet. Medicines made from amino acids, the building blocks of proteins, cannot be swallowed because they do not get across the gut wall. For example, insulin, a life-saving drug for people with diabetes, is made from 55 amino acids. Insulin is a hormone produced by the pancreas that allows cells to remove high amounts sugar in the blood after eating, and to use it for energy. People with diabetes do not make enough insulin and cannot safely reduce the sugar levels in their blood. They must get their insulin by taking it as a medicine. Unfortunately, insulin is a really large molecule, which makes it difficult to cross the gut wall to get into the blood. Instead, insulin must be injected under the skin to make its way to the blood to do its job in the liver.
Many other important drugs are also made of amino acid chains, called peptides. Large peptides are called proteins. Most peptide drugs have the same problem as insulin: they must be injected. But imagine if we could make these medicines into a pill or tablet so that they could be swallowed! Patients would be much happier to swallow them instead of injecting them. However, this is a huge challenge.
Insulin’s Horrible Journey Through the Digestive System
There are major reasons why it is not yet possible to take insulin in a tablet. First, the insulin would meet the stomach acid. Stomach acid is designed to break food down into tiny particles, so it would quickly destroy the insulin. The stomach also produces an enzyme called pepsin, which is expert at breaking down insulin. Even if the insulin were to survive stomach acid, it would encounter a new set of problems when it enters the next part of the digestive system: the small intestine. Tiny food particles broken down by stomach acid now cross the wall of the small intestine in the form of amino acids, to enter the blood. Absorbing nutrients from food is what the small intestine is designed to do. The wall of the small intestine is lined with slippery mucus—a bit like snot! Because insulin is much larger than the single amino acids released by food particles, it is really hard for it to cross this slimy lining and it is also too large to cross the gut wall. If a person were to swallow an insulin tablet, not enough insulin would be able to enter the blood and travel to the liver, and most of it would be lost on its journey. In this form, insulin has low bioavailability, which means that the amount of insulin that successfully gets into the blood can barely be measured compared to the amount in the tablet. This would be a waste of expensive medicine and it would not help in the treatment of diabetes.
The general path insulin might take as part of an effective “designer tablet” that could be taken by mouth is summarized in Figure 1. After swallowing, the tablet makes its way to the stomach where it is churned up, but eventually, in the upper part of the small intestine, it would be absorbed across the gut wall—but only if we can engineer it to do so.
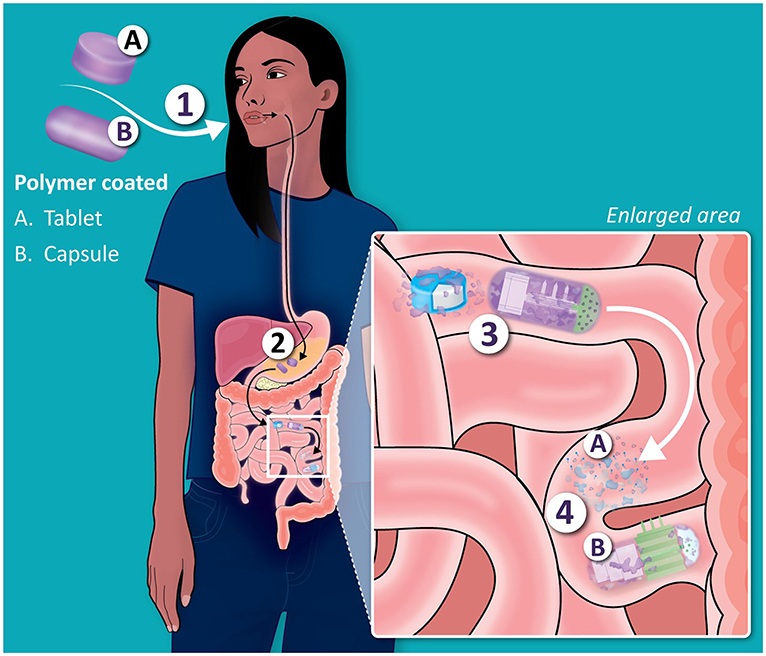
- Figure 1 - What happens when we take “designer tablets” containing insulin by mouth?
- (A) Tablet coated with a polymer permeation enhancer. (B) Microneedle capsule. (1) First, the medicine is swallowed and passes to the stomach. (2) The polymer protects the tablet/capsule in the stomach. (3) Intact tablet and capsule move from stomach to small intestine. (4) Polymer coatings (purple) dissolve from the tablet/capsule, allowing insulin to cross the gut wall, either by being absorbed or with the help of tiny needles called microneedles.
Helping Insulin Survive in the Digestive System
To get enough insulin to the liver, the first digestive system process that we must protect it from is stomach acid and pepsin. So far, scientists have been able to make a tablet that is coated with a substance that protects the insulin from stomach acid [1]. The coating is designed to disappear once the tablet leaves the stomach and enters the small intestine, so the insulin can be released there. This special coating is called a polymer. Polymers are composed of molecules bonded together in long, repeated chains, like a chain of Lego blocks. The polymer used to coat insulin tablets dissolves as the acid levels drop in the small intestine. The second digestive process we must help insulin with is crossing the lining of the small intestine, so that it can enter the blood. To do this, scientists added a substance called a permeation enhancer to the tablet. One special permeation enhancer called sodium caprate is normally found in milk. An image of how this tablet is assembled is shown in Figure 2A.
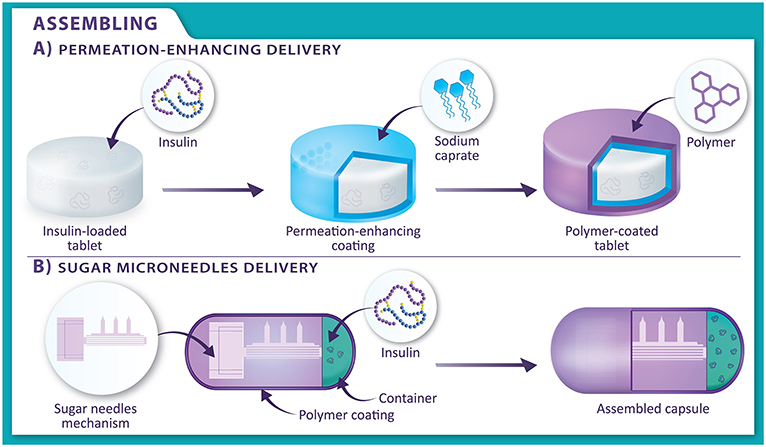
- Figure 2 - The two types of oral systems to administer insulin.
- (A) In permeation-enhancing delivery, an insulin-loaded tablet (white) is covered in a layer of a permeation enhancer (blue). The permeation enhancer is then covered in a polymer layer (purple), which provides protection from stomach acid. (B) In sugar microneedles delivery, sugar needles and insulin are put inside the middle of a capsule. The capsule is covered in a polymer layer (purple).
Scientists have worked out how sodium caprate acts on the lining of the small intestine to allow substances to safely cross the wall. Figure 3A shows how the insulin released from the tablet can cross the gut wall in the presence of sodium caprate. Using this method, only 2% of the insulin made it from a tablet to the patient’s blood [1], meaning 98% of the insulin was lost! But we are making progress. Even though the bioavailability of insulin in the tablet was so low, scientists have delivered two other peptides with other permeation enhancers in tablets to patients in 2019 and 2020 [2], and these are now available in pharmacies around the world.
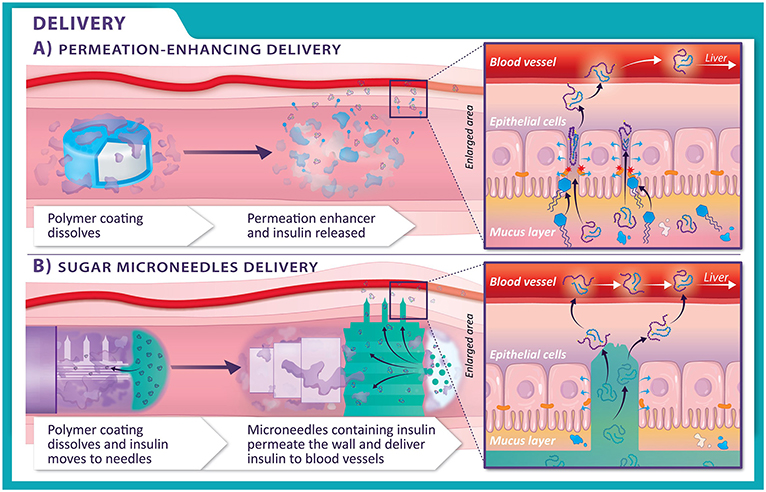
- Figure 3 - (A) In permeation-enhancing delivery, as the tablet moves from the stomach to the small intestine, the polymer layer (purple) disappears, and the permeation enhancer (blue) and insulin (white) release.
- The permeation enhancer opens up spaces between the cells, allowing the insulin to cross into the blood vessels. (B) In sugar microneedles delivery, the polymer layer (purple) disappears from the capsule in the small intestine. The microneedles, containing insulin, are activated and pop out of the capsule. The needles dig into the cells lining the walls of the intestines, allowing insulin to get into the blood vessels.
Nifty Nanoparticles
Peptides like insulin can be protected by coating them with tiny particles called nanoparticles, which are smaller than a grain of sand. The cells lining the walls of the intestines act like a vacuum cleaner to suck up these nanoparticles, though not very well! Nanoparticles can also be coated with molecules to disguise them as food, to trick the intestinal wall into taking them up. Scientists are trying to make nanoparticles made from silica [3], the main component of glass and talcum powder. Polymers are also being used to make nanoparticles. Polymers can be natural, or human made. However, if the design of the nanoparticles gets too complicated, they become far too expensive to make in large amounts. Also, only a few peptides attached to nanoparticles have ever been assessed in humans, and they failed to work. Others are being studied that can cross the slimy lining and get taken up by gut wall cells.
What About Microneedles?
Microneedles are tiny needles, thinner than a strand of hair, which can be used inside the body to help create pathways for delivery of medicines. What if we could simply get through the wall of the intestine using microneedles? Scientists are currently designing a special capsule that can carry insulin and travel unharmed through the digestive system to the gut wall [4]. The surface of the capsule is covered with microneedles, made of a type of crunchy sugar, which only appear when the capsule swells up with water (Figure 2B). The microneedles expand and pierce the gut wall, allowing the insulin (or any other peptide) to transfer through the microneedles across to the other side of the cells lining the gut (Figure 3B).
But wait! We were trying to get away from needle injections, right? Do not worry, these microneedles do not cause any pain because they are so short. Also, there are very few nerves in the gut wall. If the capsule manages to activate itself and attach to the wall, the bioavailability values seen in animal studies can be over 50%! Of course, the worry is that the needles could damage the wall of the intestine in the longer term, so more testing is needed.
No More Needles?
Designing new ways to deliver medicines safely to various parts of the body requires imagination. Finding solutions like those discussed in this article will offer patients choices other than injections, helping them manage their illnesses in a way that suits them best. The methods we described already work for a few peptides, but those examples do not require a lot of the drug to reach the blood to treat patients effectively. Insulin delivery is especially complicated because we must make sure that the tablets or pills will work properly every time. An accurate dose of insulin is extremely important so that patients keep their blood glucose levels from getting too high or too low. The efforts being made with tablets and pills, nanoparticles, and microneedles are making it more likely that, in the future, people will not need to get as many injections to treat their illnesses. Hopefully, this means that patients will find it easier to manage their illnesses and live well!
Glossary
Protein: ↑ A group of 50 or more amino acids linked together with chemical bonds. Proteins are made of longer chains of amino acids and have more complex structures than peptides.
Insulin: ↑ A hormone made by the pancreas that helps glucose (sugar) enter the body’s cells, where it can be used for energy or stored for future use.
Peptide: ↑ A short string of 2–50 amino acids linked together with chemical bonds. Peptides are made of shorter chains of amino acids than proteins. Insulin is usually called a peptide.
Bioavailability: ↑ The proportion of a drug that was originally in a tablet compared to what is detected in the blood after swallowing it. A measure of how efficient the delivery system is.
Polymer: ↑ A large molecule made up of many repeated smaller units, like a long chain built from identical links. Polymers can be natural or made in labs. Superglue is an example.
Permeation Enhancer: ↑ A substance that increases the amount of medicine that is absorbed by the body across the gut wall.
Nanoparticles: ↑ Tiny particles less than the size of grain of sand, usually up to 500 nanometres (more than several million times smaller than a meter).
Microneedles: ↑ Microneedles are long enough to deliver medicine, but small enough so they do not stimulate nerves and cause little to no pain.
Conflict of Interest
The authors CR and SG declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. DB declares that he consults for companies working on giving peptides by the oral route.
Acknowledgments
This Education and Public Engagement study was supported by the Science Foundation Ireland (SFI) Center for Medical Devices (CURAM; Grant No. 13/RC/2073_P2) and by the European Union Regional Development Fund. We thank our graphic designer, Maciek Doczyk of CÚRAM, the SFI Research Center for Medical Devices at University of Galway, Ireland, for the artwork.
References
[1] ↑ Halberg, I. B., Lyby, K., Wassermann, K., Heise, T., Zijlstra, E., and Plum-Mörschel, L. 2019. Efficacy and safety of oral basal insulin versus subcutaneous insulin glargine in type 2 diabetes: a randomised, double-blind, phase 2 trial. Lancet Diabet. Endocrinol. 7:179–88. doi: 10.1016/S2213-8587(18)30372-3
[2] ↑ Brayden, D. J., Hill, T. A., Fairlie, D. P., Maher, S., and Mrsny, R. J. 2020. Systemic delivery of peptides by the oral route: formulation and medicinal chemistry approaches. Adv. Drug Deliv. Rev. 157:2–36. doi: 10.1016/j.addr.2020.05.007
[3] ↑ Hristov, D., McCartney, F., Beirne, J., Mahon, E., Reid, S., Bhattacharjee, S., et al. 2020. Silica-coated nanoparticles with a core of zinc, l-arginine, and a peptide designed for oral delivery. ACS Appl. Mater Interf. 12:1257–69. doi: 10.1021/acsami.9b16104
[4] ↑ Dhalla, A. K., Al-Shamsie, Z., Beraki, S., Dasari, A., Fung, L. C., Fusaro, L., et al. 2022. A robotic pill for oral delivery of biotherapeutics: safety, tolerability, and performance in healthy subjects. Drug Deliv. Transl. Res. 12:294–305. doi: 10.1007/s13346-021-00938-1