Abstract
The heart is a pump that brings blood to every part of the body. Calcium plays important roles in the electrical activity and pumping function of the heart. Calcium particles enter the heart muscle cells during each heartbeat and contribute to the electrical signal that coordinates the heart's function. Calcium particles also bind to machinery within the cell that helps the cell to squeeze together (“contract”), which makes the heart pump blood. In some diseases, the doors controlling the movement of calcium malfunction, leading to abnormal electrical signals, which may cause a group of heart diseases called heart rhythm disorders. In addition, abnormal regulation of calcium may directly impair pumping function or relaxation of the heart. Scientists have identified that calcium-handling abnormalities play major roles in many heart rhythm disorders. However, despite the advancements in (bio)medical technologies, several important questions about the mechanisms and treatment of calcium-related problems remain.
Why is Calcium so Important for the Heart?
Calcium is present in most foods, notably dairy products, such as milk and cheese, and is often found in small fish and some vegetables. It has been known for a long time that calcium is beneficial for the strength of our bones. In addition, scientists have discovered that calcium also plays an important role in the heart (Figure 1). The heart beats more than 2 billion times during an average person's lifetime to circulate the blood, which is needed to provide energy to every part of the body. The heart consists, among many other things, of 3 billion heart muscle cells that squeeze together (“contract”) during each heartbeat and together are responsible for the pumping function of the heart. To make sure that each cell contracts at the right moment, the heart uses an electrical signal that moves from cell to cell, much like a wave in a stadium, where the activity of one person activates their neighbor. Research during the last decades has revealed that calcium particles are responsible for the link between electrical activation and mechanical contraction (Figure 1). Calcium particles, which have an electrical charge, enter the heart muscle cells during each beat and contribute to the electrical signal. In addition, these calcium particles initiate contraction by binding to specialized machinery within the cell. When the calcium binds, the machinery starts to move and makes the cell squeeze together. On the other hand, when the calcium particles are removed from the heart cells, this triggers relaxation, allowing the heart to be refilled with blood before the start of the next heartbeat. Thus, without calcium, our hearts would stop beating immediately, which was already shown experimentally by Dr. Sydney Ringer in the early 1880s.
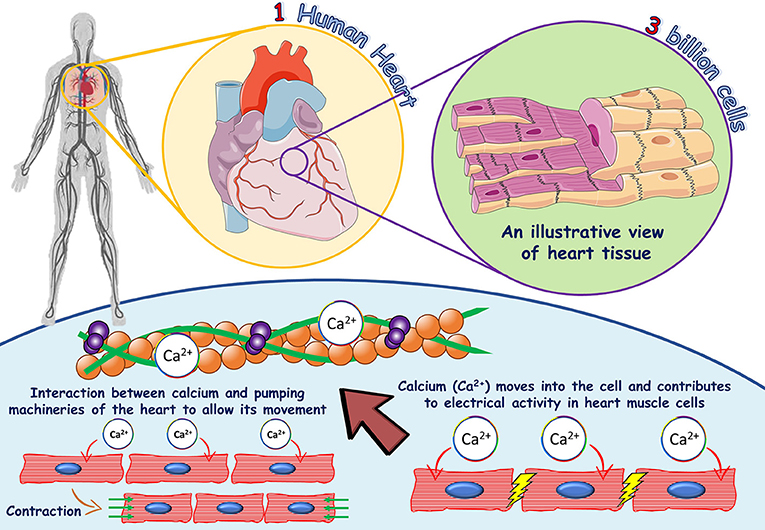
- Figure 1 - The role of calcium in heart muscle cells.
- One human heart consists of more than three billion heart muscle cells and each cell contains its own machinery to provide contraction and relaxation of the heart muscle. The blue shaded area shows the important role of calcium in heart muscle cells. Calcium binds to the troponin complex in the cells (indicated by the large red arrow), which makes the cells squeeze together, a process called excitation-contraction coupling (shown by green arrows on the left-hand side of the blue shaded area). In addition, calcium contributes to the electrical signal which moves from cell to cell to produce a uniform contraction (shown on the right-hand side of the blue shaded area).
The Heart Muscle Cell: A House With Multiple Doors and Chambers
A heart muscle cell is like a big house with multiple doors and chambers (Figure 2). Calcium particles can flow in and out of the cell through gate-like structures named ion channels [1]. These ion channels help the cell to control the amount of calcium inside of it. In addition to the supply of calcium from outside the cell, there is a big chamber inside the cell, named the sarcoplasmic reticulum, that stores most of the calcium required for heart contraction. The sarcoplasmic reticulum chamber also has entrance and exit doors for calcium. The entrance doors to the sarcoplasmic reticulum are named SERCA and the exit doors are named ryanodine receptors. The calcium that enters the heart cell through the calcium ion channel activates the ryanodine receptor to release enough calcium from the sarcoplasmic reticulum to initiate heart muscle contraction. This is done by binding to another structure, named troponin, inside the heart muscle cell. During relaxation, calcium has to be detached from troponin and expelled out of the cell or stored back inside the sarcoplasmic reticulum.
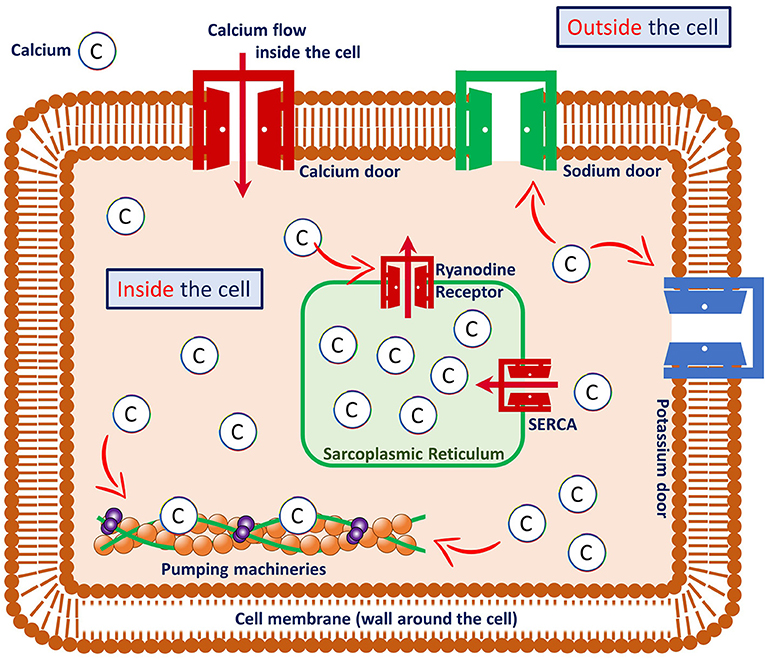
- Figure 2 - A heart muscle cell and its components.
- Calcium enters the cell through “doors” called ion channels, and interacts with various components of the cell. For example, calcium regulates the opening and closing of sodium and potassium doors and ryanodine receptors, and it binds to the troponin complex to make the heart cell squeeze together (“contract”), which produces the pumping function of the heart. In heart muscle cells, most of the calcium is stored inside a chamber named the sarcoplasmic reticulum. The calcium in the sarcoplasmic reticulum is released during heart muscle contraction and transported back inside the sarcoplasmic reticulum during relaxation. Red arrows indicate the movement/flow of calcium from one place to another.
In addition to the calcium doors, heart muscle cells are also equipped with other doors responsible for the movements of other particles in and out of the cell, such as sodium, potassium, and chloride. Recently, scientists have found that calcium can regulate the activity of these other doors, making them easier or harder to open, highlighting the large responsibility of calcium in heart muscle cells [2].
What Happens if Calcium Gets Out of Control?
In some cases, the doors controlling the movement of calcium malfunction, causing too much or too little calcium to enter the cell. Sometimes, this malfunction is caused by advancing age or other diseases. Alternatively, changes/variations in our genes (called genetic mutations) can change the shape of the ion channel which, in extreme cases, may prevent the channel from opening or closing properly. This can lead to abnormal electrical signals, which may cause a group of heart diseases called heart rhythm disorders.
Heart rhythm disorders happen when the electrical communication between cells becomes uncoordinated or when groups of cells spontaneously produce additional electrical signals. As we previously mentioned, electrical communication in the heart is similar to the wave in a soccer stadium, which also relies on clear communication. If the lights are off and the spectators cannot see each other, communication will not happen and it will not be possible to create a nice wave. The wave also only works properly if people move only when the wave reaches their seat. Uncoordinated, chaotic electrical activity of the heart is called fibrillation. Fibrillation causes the heart to pump blood ineffectively, leading to a lower energy supply to a person's organs.
In addition, abnormal calcium movement may directly impair contraction or relaxation of the heart, hindering the normal pump function. Under these conditions, the heart cells can eventually become “tired” and fail. Heart failure. can cause a wide range of problems, from mild (coughing and tiredness) to severe (shortness of breath and organ swelling). This, of course, will reduce a person's productivity. Recently, scientists found that calcium is strongly associated with the progression of heart failure. Heart failure also makes the occurrence of potentially deadly heart rhythm disorders more likely [3].
What has Been Done by Scientists so Far to Better Understand Calcium in the Human Heart?
Given the impact of heart disease, we may wonder what scientists can do to stop those diseases from occurring. For several decades, scientists have been studying the role of calcium in heart muscle cells. They now know that in some heart diseases, such as fibrillation and heart failure, abnormalities in calcium regulation play a major role [2]. Nowadays, scientists can study calcium movement by taking single heart muscle cells from animals or patients and investigating these single cells using a sophisticated method called patch clamp, which makes it possible to measure the electrical signals that pass through specific ion channels. This is done by attaching a very small glass needle (more than 20 times smaller than a single hair) to the surface of the cell. Scientists can also measure the number and location of channels inside the heart cell by attaching light-emitting indicators to the channels, which can be visualized under a microscope. More recently, scientists have started to use computer models to put all these data together, to help them predict the effect that changes in calcium regulation will have within heart cells [4].
The improved understanding of heart rhythm disorders has helped to predict which patients have a high risk of these problems and has also resulted in better therapies [5]. For example, drugs can be used to block the ion channels so that the doors stay closed and the amount of calcium inside the cell is controlled. Alternatively, specialized doctors can put a small device into the heart, through the blood vessels, to take out the heart cells that produce unwanted signals so that they no longer cause fibrillation (Figure 3). Despite the impressive advancements in (bio)medical technologies, numerous important questions about the mechanisms and treatment of calcium-related problems inside heart muscle cells remain. Several research groups across the world are working hard to answer these questions.
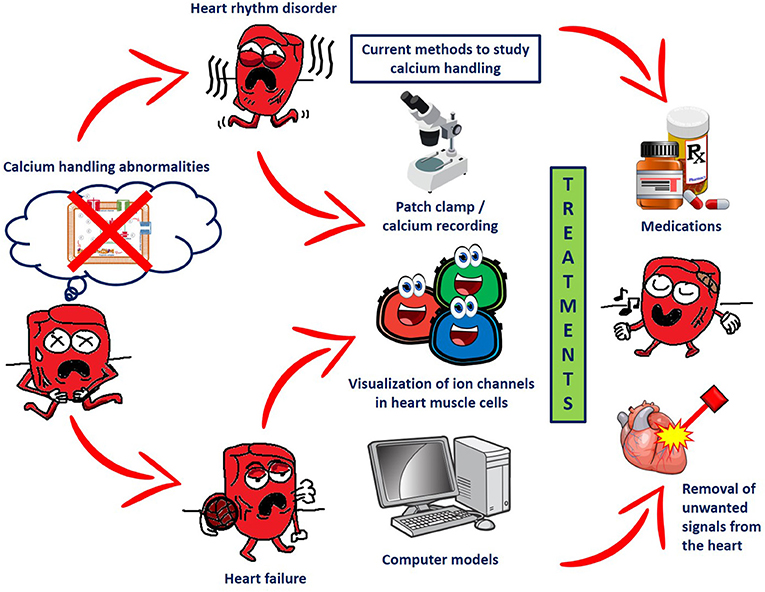
- Figure 3 - The effects of calcium-handling abnormalities in the heart and currently available methods to detect and treat these problems.
- Problems with calcium handling in heart muscle cells (shown on the left) may result in heart rhythm disorders and/or heart failure (first set of red arrows). Several methods are currently available to study the role of calcium in these diseases, including calcium recording, protein staining/coloring, and analysis with computer models (shown in the third column). Using these methods, new treatments for these heart conditions are being developed, including medications to block the ion channels and techniques to remove the cells of the heart that produce uncontrolled electrical signals (shown on the right).
Glossary
Contraction: ↑ The squeezing together of heart muscle cells, which makes the heart pump blood.
Ion Channel: ↑ Gate-like structures in heart muscle cells that allow charged particles to enter or leave the cell.
Ryanodine Receptor: ↑ An important calcium gate located within heart muscle cells on the intracellular calcium stores of the sarcoplasmic reticulum.
Genetic Mutation: ↑ A variation in a gene that may change the function of the resulting protein.
Heart Rhythm Disorder: ↑ Abnormal electrical activity of the heart.
Fibrillation: ↑ A dangerous heart rhythm disorder with very rapid irregular movement of heart muscle cells.
Heart Failure: ↑ A condition in which the heart is unable to pump sufficient blood through the body.
Patch-Clamp: ↑ A technique to measure the activity of ion channels in heart muscle cells.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
[1] ↑ Bartos, D. C., Grandi, E., and Ripplinger, C. M. 2015. Ion channels in the heart. Compr. Physiol. 5:1423–64. doi: 10.1002/cphy.c140069
[2] ↑ Heijman, J., Schirmer, I., and Dobrev, D. 2016. The multiple proarrhythmic roles of cardiac calcium-handling abnormalities: triggered activity, conduction abnormalities, beat-to-beat variability, and adverse remodelling. Europace. 18:1452–4. doi: 10.1093/europace/euv417
[3] ↑ Johnson, D. M., and Antoons, G. 2018. Arrhythmogenic mechanisms in heart failure: linking beta-adrenergic stimulation, stretch, and calcium. Front. Physiol. 9:1453. doi: 10.3389/fphys.2018.01453
[4] ↑ Sutanto, H., van Sloun, B., Schonleitner, P., van Zandvoort, M., Antoons, G., and Heijman, J. 2018. The subcellular distribution of ryanodine receptors and L-type Ca2+ channels modulates Ca2+-transient properties and spontaneous Ca2+-release events in atrial cardiomyocytes. Front. Physiol. 9:1108. doi: 10.3389/fphys.2018.01108
[5] ↑ Heijman, J., Ghezelbash, S., and Dobrev, D. 2017. Investigational antiarrhythmic agents: promising drugs in early clinical development. Expert Opin. Invest. Drugs. 26:897–907. doi: 10.1080/13543784.2017.1353601