Abstract
The gut is home to millions of microscopic organisms, which together are called the gut microbiota. Most of these microorganisms live in peace with the rest of our cells, help us get energy from food, and give us essential nutrients that we cannot make ourselves. The cells lining the gut’s surface keep these microorganisms separated from the rest of the body, like the “Great Wall” of our kingdom. On the inside of this Great Wall, the heroes of the mighty immune system watch over and protect us. But how can the immune system tell the difference between our own cells and the gut microorganisms? Or between helpful microorganisms and disease-causing ones? It is an amazing storey, full of secrets. In this article, we will explain how the gut coaches the immune system to recognise enemies, and how some aspects of modern life might be interfering with this process.
The Gut and the Microbiota: An Old Friendship
Once upon a time…the gut: it is a long tube inside the body, from mouth to bum, which includes the stomach and the intestines. These are some of the most ancient organs in our evolution, and they are experts in providing food and water to the rest of our cells. But even these “masters of feeding” cannot make all the nutrients and energy that animals need to survive. During the course of evolution, microorganisms began to live in the guts of animals, which provided a perfect home for them: warm, full of food, and protected from the outside world. The animals also benefitted from housing these microorganisms because bacteria can help break down food and can make the nutrients that the animals’ guts could not provide [1]. The entire group of microorganisms that live in the gut is called the gut microbiota. The friendship between the gut and the gut microbiota helps humans to stay healthy.
Our Immune Cells: The Body’s Superheroes
When we eat and drink, the substances entering the gut may bring in potential enemies, such as disease-causing bacteria and viruses. The cells of the immune system protect us from these invaders by fighting off infections (Figure 1) [2]. However, it is important that the immune cells do not attack friendly cells. Otherwise, the immune system would kill our own cells and our allies, the gut microbiota. This knowledge is their real superpower, something that they can only acquire with effort, study, and practise.
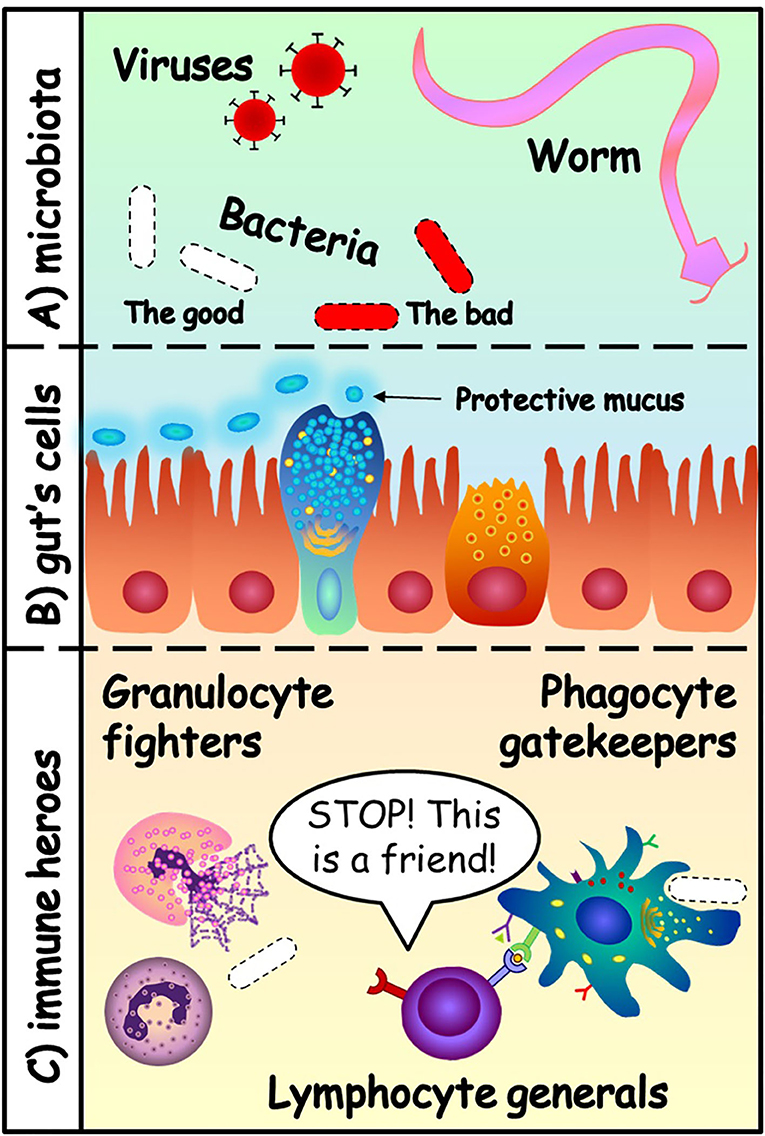
- Figure 1 - (A) The gut microbiota can contain viruses, bacteria and even worms.
- Some of these microorganisms are our friends (white), and some are dangerous (red). (B) The cells of the gut protect themselves with a layer of mucus. They form a “Great Wall” that separates two kingdoms: the microbiota and the inside of the body. (C) The heroes of the immune system protect the body: granulocytes (first-line fighters), phagocytes (gatekeepers and teachers of lymphocytes) and lymphocytes (the generals that distinguish enemies from friends).
The immune system is like a big army with many specialised soldiers. It has fighters, called granulocytes. They are our first line of defence, fast and strongly armed with toxic granules that they hold inside until they recognise the enemies. Granulocytes do not live very long and need limited training because they are born ready to attack the enemy. The toxic granules they release attack everything around them, so they could also damage friendly cells. Next, we have the gatekeepers, called phagocytes, that can chew up invading organisms. They are important for identifying whether a microorganism is good or bad. Once they do so, the phagocytes alert the rest of the immune cells and teach them to recognise the bad microorganisms.
The generals of the immune system’s army are called lymphocytes. Lymphocyte generals get training from the phagocyte gatekeepers, to learn how to coordinate the army. Some lymphocytes become experts in recognising specific enemies and help to accelerate the attack. Other lymphocytes learn to recognise friends and send the message to stop the troops from attacking. Thanks to the lymphocytes, the immune system can learn from the first fight with an infectious microorganism and respond faster against the same invader the second time it attacks. This is called immunological memory, and it is why vaccinations are so important. Some enemies are really powerful, so we do not want the immune system to face these battles without the proper training. Once they have been trained, the cells are armed and ready to protect us against infectious enemies that we have not met yet. That is the problem we had in 2020, when many people got infected with a new virus, the coronavirus. We were not ready. This is how the vaccines can help, by training the lymphocyte generals. The end of this war is coming!
A School Day in the Gut
The gut, with all its different microorganisms, is the best school for training the immune system. The cells that line the gut keep the microorganisms separated from the rest of the body, but they also protect themselves by covering their surfaces with protective sticky mucus. From the safety of this Great Wall of the gut, the immune system heroes study the microbiota [3]. Sometimes, the gut cells show the immune cells some of the microorganisms living on the other side of the Great Wall. Other times, the immune cells extend their arms through the wall to study the microorganisms trapped in the mucus (Figure 2A). This is how the immune cells become smarter, stronger, and prepared to fight any danger. We do not see or feel this, but there is always a fight about to start when the immune system recognises a microorganism at the Great Wall. However, most of the gut microorganisms are good ones and the lymphocyte generals know this, so they calm down the fighters. In addition, as long as the microbiota and the rest of the body remain separated by the Great Wall, peace reigns within the gut.
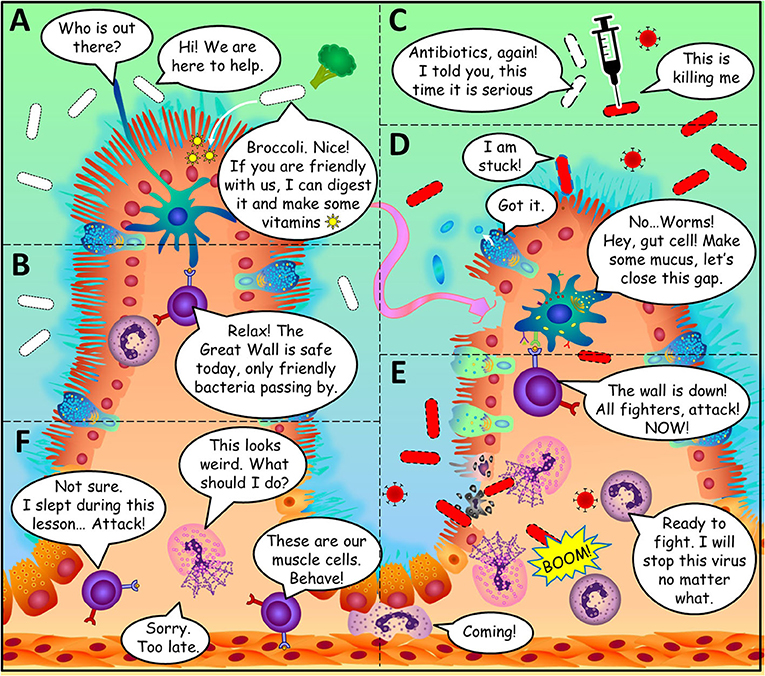
- Figure 2 - Amazing storeys happen in the gut.
- (A) Phagocyte gatekeepers can recognise good bacteria by the peace-keeping molecules they produce. (B) Lymphocyte generals can calm down the fighters if the microorganism is a friendly one. (C) Antibiotics help kill disease-causing bacteria, but they can also kill the good ones. (D) The gut cells form a barrier to keep microorganisms out of the body. (E) The immune cells come to the rescue when the barrier is breached. (F) Unfortunately, some aspects of modern life can cause immune cells to fight against the body’s own cells.
How do the cells of the immune system distinguish body cells and good microorganisms from bad microorganisms? Our body cells are easy to recognise because they all wear the same molecules on their surfaces, the way a soccer team wears jerseys of the same colour. These molecules are very different from those on the surface of microorganisms. The granulocyte fighters and phagocyte gatekeepers can detect the specific molecules on microorganisms. However, good and bad microorganisms have very similar molecules on their surfaces, so it is quite difficult to tell the good guys from the bad guys within the microbiota. Amazingly, the immune system can tell these microorganisms apart because some good bacteria in the microbiota produce molecules that serve as a message of peace to the immune system. These molecules are the way microorganisms say, “Hello, friend, I am here to help!” (Figure 2). The gut is full of secrets messages like that one and scientists are working to discover them all.
Modern World Diseases: The Superheroes’ Weakness
Sometimes things do not go quite right, and the gut can become infected. Years ago, sanitary conditions were worse than today: houses were not as clean, and food and water were more often contained with disease-causing microorganisms. That means that people got sick more often from ingesting bad microorganisms. Every time they got sick and recovered, their immune systems became better trained at fighting them. Even when they got infected by microorganisms that resisted the fight, such as worms, the immune system learned instead to repair the damage that they caused [4]. So, one way or another, the people could recover from future infections faster.
Today, our lifestyles are very different and science has solved many of the problems of the past. We have antibiotics to kill the enemies that infect us. Our homes, foods, and water are cleaner and less likely to make us sick. Together, these changes modify the gut’s environment, the microbiota, and the immune system. Now, dangerous enemies do not have as many opportunities to infect us as they once did. But it is also a bit “boring” for the immune system, which is an important new problem. Our microbiota contains fewer enemies, but also fewer types of good bacteria. When we take antibiotics, our good bacteria are killed along with the disease-causing bacteria. By eating different foods than our ancestors ate, we are also changing what our microbiota eats. All these changes are misleading our immune system superheroes, by interfering with their training.
The combination of all these small lifestyle changes has produced bored and confused immune cells that are up to no good: they start fighting and never know when to stop. Sometimes they even attack the body’s own cells. The undertrained superheroes become villains. This has resulted in new types of diseases, including allergies, inflammatory bowel disease, arthritis, psoriasis, diabetes, obesity, depression, cardiovascular diseases, and even cancer. Interestingly, when scientist started studying these “modern” diseases, they discovered that many of them are connected to the immune system heroes and their training camp, the gut [5]. These modern diseases are very complex because, even in groups of people who share the same lifestyle, not everyone becomes ill. This happens because all individuals are different, not only in traits like hair colour or height, but also in the way their cells work.
Conclusion
The world is changing, for us and for our gut microbiota. But do not worry! There are some things you can do to take care of your gut microbiota so that it continues to effectively train your immune system. Bad bacteria really like sweets, so exchange sugary foods for fruits and veggies when you can. Natural, unprocessed foods will also help your good bacteria grow and protect you. Be careful with antibiotics! Only take them when recommended by your doctor. Wash your hands before eating, but do not become obsessed about cleanliness, this is enough to avoid most of the infections. If you do these things, there is no need to worry—you can trust your immune superheroes. And remember, like the immune system, we all have the abilities to become heroes and the weaknesses to become villains. The efforts that we make to learn and improve are what makes the difference. How wonderful the world would be if we could all learn from the training ground of the gut!
Glossary
Gut: ↑ The part of the body involved in processing food, consisting of a long tube that goes from the mouth to the anus, and the organs that collaborate in this function.
Microbiota: ↑ The community of helpful microorganisms found in the body, such as virus, bacteria, and fungi.
Immune System: ↑ The group of cells that maintains the health of the body by protecting us from infections.
Granulocytes: ↑ The first line of defence of the immune system. These cells are the fastest fighters, strongly armed with toxic granules to attack the infectious enemies.
Phagocytes: ↑ These cells are the teachers of the immune system. They can chew up invading organisms and teach them to recognise the bad microorganisms.
Lymphocytes: ↑ These are the generals of the immune system’s army. They become trained by the phagocyte gatekeepers to remember all the microorganism and coordinate the response of the immune system.
Immunological Memory: ↑ The ability of the lymphocytes to recognise and remember all the cells and the microorganisms that they have encountered before. This is how they become better at protecting the body.
Antibiotics: ↑ Medicines used to fight infections caused by bacteria.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank Alba Bosquet Agudo and Shani Naomi Austin-Williams for a critical review of this manuscript.
References
[1] ↑ Da Silva, G., and Domingues, S. 2017. We are never alone: living with the human microbiota. Front. Young Minds. 5:35. doi: 10.3389/frym.2017.00035
[2] ↑ Lundy, S. 2018. The immune system, in sickness & in health—part 1: microbes and vaccines. Front. Young Minds 6:49. doi: 10.3389/frym.2018.00049
[3] ↑ Yilmaz, B., Carvalho, J., and Marialva, M. 2019. The intestinal universe—full of gut heroes who need sidekicks. Front. Young Minds 7:111. doi: 10.3389/frym.2019.00111
[4] ↑ Tunnessen, N., and Hsieh, M. 2018. Eating worms to treat autoimmune diseases?. Front. Young Minds 6:32. doi: 10.3389/frym.2018.00032
[5] ↑ Lima-Ojeda, J., Rupprecht, R., and Baghai, T. 2019. Happy gut bacteria, happy brain: the microbiota-gut-brain axis. Front. Young Minds 7:15. doi: 10.3389/frym.2019.00015