Abstract
Today, pretty much everyone knows that burning fossil fuels is bad for the environment, and that new approaches are needed to provide environmentally friendly energy resources for the world’s growing population. One promising solution involves producing “green” hydrogen fuel, by using interesting materials called photocatalysts to break apart water molecules—H2O—into hydrogen and oxygen, using the energy of sunlight. This process is called photocatalytic water splitting, and it is an exciting area of research. In this article, we will describe our photocatalytic water splitting system, which involves coating large glass sheets with water-splitting materials, assembling them into panels, adding water, shining light on them, and collecting the hydrogen that is produced. Although our initial outdoor experiments are very promising, there is still important work to be done. We will explain some of the challenges that must be overcome before this strategy can be used to produce power on a large scale.
Could Water Be the Secret Ingredient for Future Energy?
Water—everyone knows that it is important for things like drinking, cooking, and keeping plants alive, right? But what if we told you that this seemingly simple, tasteless, colorless liquid could be the key to powering cars, homes, industries, and maybe even entire cities in the future? This is not just a wild idea; it is a real scientific breakthrough called photocatalytic water splitting, in which scientists use sunlight to transform water into a clean, “green” fuel.
Fossil fuels like coal, oil, and natural gas have powered our world for centuries, providing the energy needed for everything from lighting and heating our homes to running our appliances and electronic devices. However, burning fossil fuels releases a significant amount of carbon dioxide (CO2) and other pollutants into the atmosphere. These gases trap heat, leading to global warming and climate change, which are causing an increase in extreme weather events, rising sea levels, and widespread damage to the environment… and climate change and air pollution are even making some people sick! On top of that, fossil fuels are not limitless resources. As we continue to extract them from the Earth and use them, they are becoming harder to find and more expensive to produce.
These problems have created an urgent need for “greener” sustainable energy sources that can provide a steady supply of energy without harming the environment. Solar energy, wind power, and biofuels are all part of the solution, but one of the most promising alternatives is hydrogen fuel, particularly when it is produced in an environmentally friendly way.
The Promise of Hydrogen Fuel
Hydrogen is the most abundant element in the universe, and it has great potential as a clean fuel. To generate energy from hydrogen, it is fed into a device called a fuel cell. You can think of a fuel cell as a kind of battery that never runs out, as long as it has a supply of hydrogen. Inside a fuel cell, hydrogen reacts with oxygen to produce electricity. Specifically, hydrogen molecules are split into protons and electrons. The electrons travel through a circuit, creating an electric current, while the protons combine with oxygen molecules and electrons coming back from the circuit to form water molecules—the only byproduct. This makes hydrogen fuel incredibly clean and environmentally friendly, offering a solution to reduce greenhouse gas emissions significantly.
But first things first—where do we get the hydrogen? The way most hydrogen is produced today relies on natural gas, which is a fossil fuel. Through several steps, natural gas is heated with steam to produce hydrogen and CO2, so this process still adds to fossil fuel use and greenhouse gas emissions. To make green hydrogen, we need to produce it using renewable energy sources, not fossil fuels. This is where water and sunlight come in! By using sunlight to split water molecules into hydrogen and oxygen, we can produce hydrogen in a way that is both sustainable and environmentally friendly. This method is called photocatalytic water splitting—“photo” means “light” and “catalytic” describes a substance that speeds up a chemical reaction (like water splitting). Photocatalytic water splitting could someday transform our energy system by providing a virtually limitless supply of clean fuel.
As a fuel, green hydrogen can be used in many ways, such as powering cars and homes or as an important part of industrial processes. It can also help to store solar energy as hydrogen fuel. The energy can be used later when the sun is not shining. Imagine a future in which cars run on hydrogen fuel, producing only water as exhaust, or where entire cities are powered by hydrogen produced from sunlight and water, without releasing any smog or greenhouse gases. This is the potential future that photocatalytic water splitting aims to achieve.
But How Does It Work?
Photocatalytic water splitting uses sunlight to break down water (H2O) into its basic components: hydrogen (H2) and oxygen (O2). The key to this process is using materials known as photocatalysts. These materials can absorb sunlight and use that energy to break the chemical bonds in water molecules. Photocatalysts are usually made from semiconductor materials like solar cells that can efficiently harness solar energy, but they need to be stable enough to work properly in water.
There are several types of photocatalytic systems. One common approach is to use photocatalysts in a powdered form, mixed into water in a big, clear tank (Figure 1A). When sunlight hits the water, the photocatalyst particles absorb the light and generate the energy needed to split the water molecules. Another approach involves using thin films of photocatalysts coated on various surfaces (Figure 1B). These films are usually submerged in shallow trays filled with water and placed in direct sunlight to produce hydrogen. In either system, the hydrogen that is produced rises to the surface and can be collected using a series of tubes or gas collection chambers, and it is stored in tanks for later use.
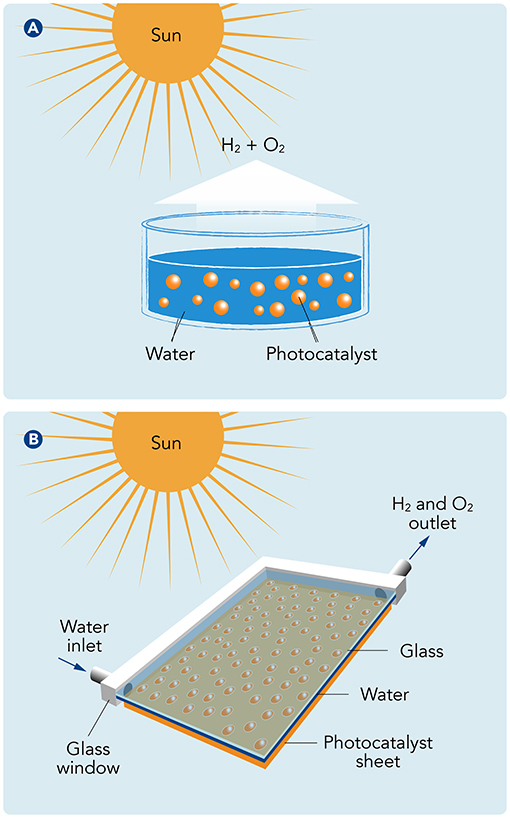
- Figure 1 - (A) In one type of photocatalyst system, a powdered photocatalyst is mixed with water in a clear tank.
- When sunlight enters the tank, it gives the photocatalyst the energy to split water into hydrogen and oxygen. (B) We made our system by coating a thin film of photocatalyst onto large glass sheets that were assembled into panels. The panels have an inlet where water can come in and an outlet through which hydrogen and oxygen can leave.
Each system has its own advantages and challenges. For example, powdered photocatalysts can be easily mixed into large volumes of water, making it possible to evenly distribute the photocatalyst particles at a high enough concentration to absorb sunlight and to quickly release the gases that are produced. However, collecting and reusing the powdered catalysts can be difficult. Thin films, on the other hand, are easier to handle and reuse, but they have to be designed very carefully to make sure they receive enough light.
Building a Photocatalytic System
Because thin films are easier to use and can be reused, we decided to create a system based on that method. We hoped to design a system that could work on a large scale, and out in the real world instead of in a lab. These qualities are necessary if we wish to use photocatalytic systems to produce enough hydrogen to meet some of the world’s energy demands and help reduce our reliance on fossil fuels.
In our research, we coated photocatalysts onto large glass sheets. These photocatalyst sheets were then assembled into panels that look kind of like the solar panels you have probably seen in fields or on people’s houses. We set up the panels outdoors, where they could receive plenty of sunlight, and added water (Figure 2) [1]. When sunlight hits these panels, the photocatalysts absorb the light and generate the energy needed to split water molecules into hydrogen and oxygen (which are both gases at room temperature). We used tubes and storage tanks to collect the hydrogen gas produced by our system, and we released the oxygen into the atmosphere.

- Figure 2 - An aerial photo of a 100-square-meter outdoor panel reaction system for hydrogen production using sunlight for photocatalytic water splitting.
One of the key aspects of building photocatalytic water splitting systems that can be used in real life is ensuring that they can withstand the weather. To test this, outdoor experiments are better than lab experiments, because they expose the panels to varying weather conditions, from intense sunlight to rain and wind, and from scorching summer to freezing winter. Our initial experiments have been very promising, showing that our system can produce hydrogen efficiently under real-world conditions. This is an exciting step toward making photocatalytic water splitting a useful way to produce lots of hydrogen for everyone to use.
Important Work Remains…
While our initial results are promising, several big challenges must still be addressed before photocatalytic water splitting can be used on a large scale [2]. One big challenge is finding the right materials to efficiently absorb sunlight and catalyze the water-splitting reaction. Currently, even the best photocatalysts can convert only about 1–2% of the sunlight they receive into hydrogen. To make this technology affordable, we need to increase this efficiency to at least 10%. With this improved efficiency, the ground area needed to meet our energy demand is 800,000 m². This is much larger than the world’s largest solar power plant in Sweihan, United Arab Emirates (7.8 km²), and even larger than the area of Japan (378,000 km²) or the United Kingdom (244,000 km²). So, we need to come up with new technology to build, run, and maintain these large photocatalyst panel reactors. To do this, scientists are constantly experimenting with different materials and methods, to try to make the best photocatalysts and water-splitting systems [3].
Safety is an extremely important consideration. The process of splitting water initially produces a mixture of hydrogen and oxygen gases, which must be separated to obtain pure hydrogen. When hydrogen and oxygen are combined, the mixture is highly flammable and can be explosive if not handled properly. Scientists must be extremely careful to follow all laws and regulations, to keep the entire process as safe as possible and avoid dangerous accidents. One solution might be to design systems that can safely separate the hydrogen from the oxygen as soon as they are produced, so a mixture is never formed. This would help to prevent fires or explosions because these gases are not explosive on their own.
Finally, photocatalytic water splitting systems are still very expensive to build and maintain. Right now, it is much cheaper to generate hydrogen from fossil fuels than from water splitting. Currently, hydrogen produced from photocatalytic water splitting can cost more than hydrogen produced using methane, for example. So, scientists need to figure out how to keep the costs down, so that these systems are competitive with other energy sources—otherwise, people will probably not choose to use them.
The Future of Green Hydrogen
Despite the challenges that remain, the future of photocatalytic water splitting looks bright. If we can safely scale up this technology, it could provide a virtually limitless supply of clean fuel. This would help reduce our reliance on fossil fuels and significantly cut down on greenhouse gas emissions, protecting our planet and all its inhabitants. This vision of a cleaner, greener future is what drives scientists and engineers to keep trying to develop new materials and techniques that will improve green hydrogen production. So, the next time you take a sip of water, remember that this simple liquid might just be the key to powering our future—how cool is that?
Glossary
Photocatalytic Water Splitting: ↑ A reaction in which water molecules are broken down into hydrogen and oxygen molecules, using materials called photocatalysts and the help of light energy.
Sustainability: ↑ A concept for keeping people’s lives, society’s functions, and the planet’s environment in good shape over the long term.
Green Hydrogen: ↑ Hydrogen made with no CO2 emissions, using renewable energy sources.
Renewable Energy: ↑ Types of energy that are always replenished by nature and will never run out, unlike fossil fuels. These include solar, wind, wave, tidal, water current, tides, geothermal, and biomass.
Photocatalysts: ↑ Substances that absorb light and promote a reaction using light’s energy.
Semiconductors: ↑ Substances that conduct electricity under certain conditions. They fall between conductors like metal and insulators like rubber. In photocatalysis, they absorb sunlight to break down water into hydrogen and oxygen.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Edited by Susan Debad, Ph.D., graduate of the University of Massachusetts Graduate School of Biomedical Sciences (USA) and scientific writer/editor at SJD Consulting LLC. We would like to thank the coauthors of the original manuscript, Qian Wang of Nagoya University, Fuxiang Zhang of Dalian Institute of Chemical Physics, Shane Ardo of University of California Irvine, Erwin Reisner of University of Cambridge, Hiroshi Nishiyama, Taro Yamada of The University of Tokyo, and Akihiko Kudo of Tokyo University of Science, for their contributions and discussion. KD thanks the New Energy and Industrial Technology Development Organization (NEDO, project no. P21021) for financial support. TH thanks the Japan Science and Technology Agency (JST, grant no. JPMJPR20T9) for financial support.
Original Source Article
↑Hisatomi, T., Wang Q., Zhang, F., Ardo, S., Reisner, E., Nishiyama, H. et al. 2024. Photocatalytic water splitting for large-scale solar-to-chemical energy conversion and storage. Front Sci 2:1411644. doi: 10.3389/fsci.2024.1411644
References
[1] ↑ Nishiyama, H., Yamada, T., Nakabayashi, M., Maehara, Y., Yamaguchi, M., Kuromiya, Y., et al. 2021. Photocatalytic solar hydrogen production from water on a 100-m2 scale. Nature 598:304–7. doi: 10.1038/s41586-021-03907-3
[2] ↑ Hisatomi, T., and Domen, K. 2023. Overall water splitting: what’s next? Next Energy 1:100006. doi: 10.1016/j.nxener.2023.100006
[3] ↑ Takata, T., Jiang, J., Sakata, Y., Nakabayashi, M., Shibata, N., Nandal, V., et al. 2020. Photocatalytic water splitting with a quantum efficiency of almost unity. Nature 581:411–4. doi: 10.1038/s41586-020-2278-9