Abstract
Do you ever wonder how people in the driest parts of the world survive? Where does their water come from, and how do they grow their plants? They cannot rely on rain because it is so rare. Maybe they use underground water wells, but wells often dry up and are not sustainable for a large population. How do these people stay alive? The answer is desalination, a process that turns salty seawater into clean drinking water. But this life-saving process uses a lot of energy and creates lots of bad emissions that can harm the environment. Fortunately, by using a special tool called life cycle assessment, we can understand how bad desalination is for the environment and learn how to reduce its effects. This article will talk about desalination, its environmental side effects, and how we use life cycle assessments to make desalination more environmentally friendly.
Distribution of Water Around the World
Although most of the Earth is covered by water, almost 97.5% of it is seawater, meaning only 2.5% is fresh and potable (drinkable) water. Furthermore, most of this freshwater (80%) is frozen in glaciers, meaning that only 0.5% of the world’s water is potable! This tiny amount of water is spread unevenly across the Earth, and some places have much less water than others. In the drier parts of the world, such as the Middle East and North Africa, barely any water is stored in the ground. The water reservoirs are rare and cannot support a large population for a long time, because they will be used up faster than they can be filled again. In fact, so much groundwater has been sucked up by humans (around 2 trillion tons between 1993 and 2020) that the tilt of the Earth has changed, and the North Pole has been shifting at a speed of 4.36 centimeters per year!
What is Desalination and How Does it Work?
Let us break down the word desalination: “de” means removal and “saline” means salt, so desalination is the process of removing salts from saltwater to form fresh water. While this may seem like a modern technology, humans have been desalinating water for centuries. In fact, there is an account by Alexander of Aphrodisias from 320 BC (around 2,300 years ago) mentioning that sailors would boil seawater and use a sponge to absorb the water vapor, which was then squeezed for drinking [1]!
Desalination can be used to produce fresh water from salty water in two main ways. One is membrane desalination, which uses a special filter to separate salt from water. The other is thermal desalination, which uses heat to evaporate water (like the ancient sailors did).
Most types of membrane desalination use a process called reverse osmosis (Figure 1A). Reverse osmosis works by separating salty seawater and fresh water with a membrane. The membrane has tiny holes that water can fit through, but salt cannot. Pressure is applied to the salty water, pushing the water through the membrane and leaving the salt behind. This results in one side of the membrane containing fresh water and the other side containing very salty water. Thermal desalination works similarly to the water cycle. Water is boiled until it evaporates, leaving behind salt. The resulting water vapor is then cooled and condensed (like rain), and fresh water is formed (Figure 1B).
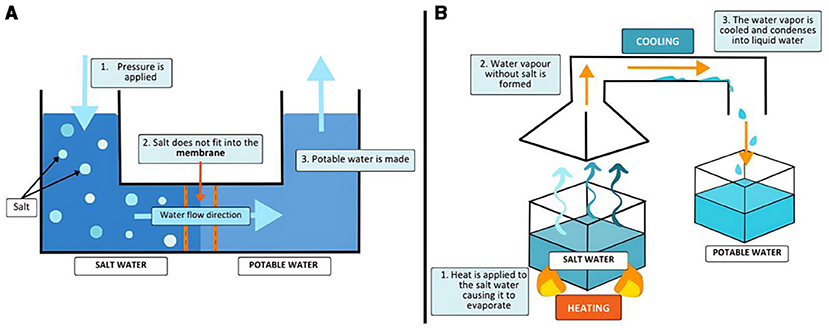
- Figure 1 - (A) In reverse osmosis, a special filter (membrane) is used to separate salt from water.
- The filter has tiny holes that allow only clean water to pass through. This gives us fresh water that we can drink or use. (B) In thermal desalination, the salty water is heated until it turns into steam. The steam rises, leaving the salt behind. Then the steam is cooled down and changes back into fresh water.
Reverse osmosis is the most common desalination technology due to its lower energy needs and costs. However, it faces challenges when it comes to very salty water, like the Red Sea. This is because too much salt can block the membrane. For saltier water, thermal desalination works better. But new technologies are changing this trend. Most new desalination plants now use reverse osmosis instead of thermal methods.
Environmental Impacts of Desalination
All desalination processes are energy intensive. The saltier the water, the more energy is required to remove the salt. To desalinate 1,000 liters of water, around 12.6 kg (28 lbs) of carbon dioxide are produced [2]. Considering that the average person uses about 382 liters of water each day, this may not seem like much. But agriculture and farming are extremely water intensive—they use almost 65% of a country’s water! Therefore, to produce enough food for just one person’s daily intake, 3,000 liters of water are needed. This means an average of 42.6 kg (94 lbs) of carbon dioxide is released per person per day, just to generate clean water!
Additionally, many dangerous chemicals are needed throughout the desalination process, which can become very harmful especially once they are disposed of. For example, a chemical called an anti-foaming agent is used to prevent seawater from foaming up, which disturbs the desalination process. Chlorine is also used, like it is in swimming pools, to disinfect the water.
A significant problem with desalination is brine. Brine is highly concentrated salt water, formed when salt is removed from seawater. Brine is pumped back into the sea, often mixed with the chemicals used in desalination. Even after treatment, diluted brine is still harmful to marine life. The excess salt and chemicals near desalination plants cause many marine organisms to die, as they cannot tolerate the higher salt levels and chemicals. In shallow seas, like the Gulf in the Middle East, there are fewer waves and less sea circulation. This causes salt to build up in dangerous concentrations when brine is dumped, which worsens the harm to marine life over time. Luckily, some new desalination plants are designed for lower brine discharge.
Desalination may seem like an excellent solution to the water problem. However, now you can see that it has serious impacts on both the environment and marine life. How can we help reduce these effects?
How Can Life Cycle Assessments Improve Desalination?
Earlier, we mentioned that reverse osmosis requires less energy compared to thermal desalination. This means it is less harmful to the environment. But how do we calculate how bad a desalination plant is for the environment? We can use a special tool called life cycle assessment. Life cycle assessment helps measure the impact a process or product has on the environment. For example, we can measure how bad desalination is for the environment by predicting the emissions produced by the plant throughout its life. Using special software, we enter all the steps and details in the desalination process. We mention the type of fuel used (e.g., fossil fuels or wind energy), the machinery, and the types and amounts of chemicals used. The software then tells us the amount and types of emissions produced (Figure 2), as well as what the emission’s final effect is.
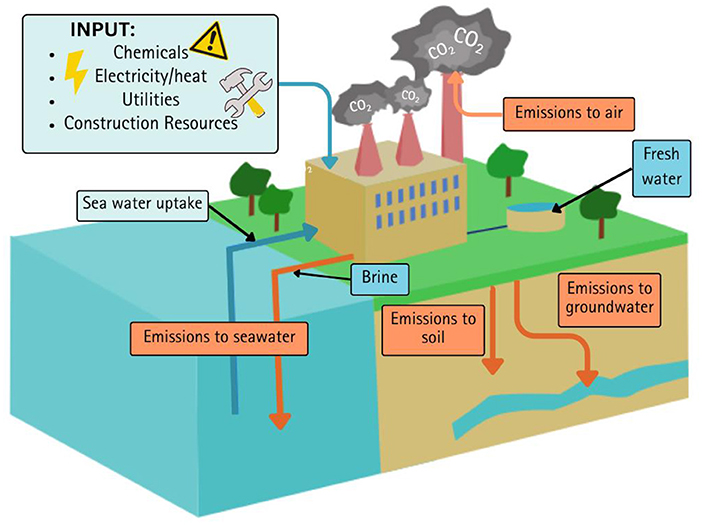
- Figure 2 - Effects of desalinating seawater.
- In each step of desalination, waste products are produced and some of them can damage the environment. Life cycle assessment can measure the amounts and types of waste products produced in desalination, to understand how bad desalination is for the environment.
For instance, if fossil fuels are used to heat seawater, a lot of carbon dioxide is produced, which contributes to climate change. If a lot of chemicals are used, such as anti-foaming agents, they will cause ozone depletion because they can damage the ozone layer high above Earth. Impacts such as ozone depletion, which are calculated by the software, are called midpoints. By using life cycle assessments, we find out which steps of desalination release the most emissions and then find alternatives to those steps, which can reduce the effects of those midpoints.
For example, Figure 3 shows the result of a life cycle assessment that compared a desalination plant using solar energy to a traditional one using fossil fuels [3]. After entering all the necessary information into the software, results showed that using solar energy instead of fossil fuels could reduce CO2 emissions by 10 kg for every 1,000 liters of water produced. That is a 78% reduction. This means the solar-powered plant, which has lower emissions, has less impact on climate change than the traditional desalination plant. The solar-powered plant’s impact on human toxicity (the effect it has on human health) was three times less harmful than that of the normal desalination plant. This is because producing the fossil fuels used by normal desalination plants releases lots of toxic emissions that harm humans. This is not the case for solar-run plants. Both plants use the same kinds and amounts of chemicals, such as anti-foaming agents, and their effects on ozone depletion are almost the same. However, the solar plant has a slightly higher impact because of the construction of the solar farms. Scientists are working on improving solar technology to make it even more eco-friendly, such as by using more environmental friendly materials and recycling old solar panels.
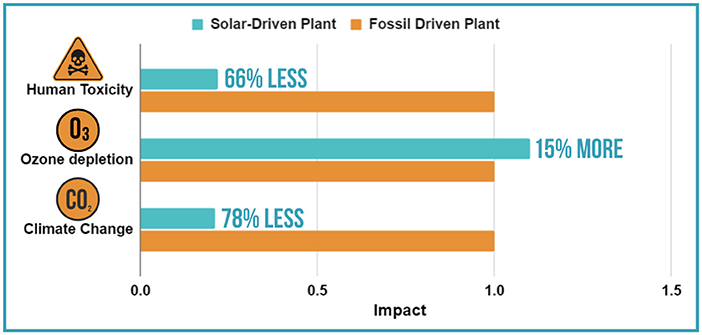
- Figure 3 - The impact of a normal desalination plant compared to one run using solar energy.
- You can see that the solar-powered plant has 78% less CO2 emissions. This means it is much better for the climate. The solar plant was also three times less harmful to human health because it does not release as many toxic gases as fossil driven plant. Both plants use the same chemicals, so their effect on the ozone is similar. The solar plant has a slightly higher impact, 15% more (figure adapted from [3]).
This life cycle assessment showed that simply by switching to solar energy instead of fossil fuels, the environmental impact of all aspects of desalination plants, except ozone depletion, is much lower. Life cycle assessments can thus not only measure the impact something has on the environment but can also be used to reduce its effects.
Conclusion
Even though desalination has many negative environmental effects, like the release of brine, chemicals, and CO2, it is still the main way millions of people around the world get clean water. However, we can significantly reduce the environmental impact of desalination using life cycle assessment! For instance, the study we described in this article compared solar energy to fossil fuels. It showed that by just transitioning to solar power, carbon dioxide emissions could be reduced by 78%. These results show the potential of using life cycle assessment in estimating environmental impacts. This analysis can help governments and engineers to plan new policies and projects in all kinds of industries, all over the world.
Glossary
Potable: ↑ Safe to drink.
Desalination: ↑ A way removing salt from seawater to make it clean and safe to drink.
Reverse Osmosis: ↑ A way to clean salty water using a special filter (membrane).
Brine: ↑ Very salty water.
Life Cycle Assessment: ↑ The analysis of the environmental impact a product or process has throughout its life.
Ozone Depletion: ↑ The thinning of Earth’s ozone layer due to the release of certain chemicals.
Human Toxicity: ↑ The potential harm a chemical has on human health.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
[1] ↑ Nair, M., and Kumar, D. 2013. Water desalination and challenges: the Middle East perspective: a review. Desalinat. Water Treat. 51:2030–40. doi: 10.1080/19443994.2013.734483
[2] ↑ Mannan, M., Alhaj, M., Mabrouk, A. N., and Al-Ghamdi, S. G. 2019. Examining the life-cycle environmental impacts of desalination: a case study in the State of Qatar. Desalination. 452:238–46. doi: 10.1016/j.desal.2018.11.017
[3] ↑ Alhaj, M., Tahir, F., and Al-Ghamdi, S. G. 2022. Life-cycle environmental assessment of solar-driven Multi-Effect Desalination (MED) plant. Desalination. 524:115451. doi: 10.1016/j.desal.2021.115451