Abstract
Have you ever heard of a beaked whale? These fascinating but mysterious whales live far away from shore and stay under water for a long time, so it is challenging for scientists to learn about them. Some species of beaked whales are so mysterious that scientists have never even seen them alive! To help learn about beaked whales, scientists can study special chemicals, called stable isotopes, in the tissues of these whales to learn where they have been living. These whale detectives work with museums and coastal communities to get tiny pieces of whale skin, muscle, and bone, which the detectives then examine for stable isotopes. Using these chemistry clues, the whale detectives discovered that some beaked whales live in one area their entire lives. The whale detectives can use this information to find and study beaked whales in the wild and answer more questions about their lives.
Beaked Whales Live Mysterious Lives
Take a moment and imagine a whale—maybe you picture a humpback whale, or a killer whale, or maybe even a bottlenose dolphin. These cetaceans (the group of animals that includes whales, dolphins, and porpoises) come to mind easily because we see and hear about them in books, movies, and the news. But have you ever heard of a beaked whale (Figure 1)? Most people have not heard about beaked whales because they live mysterious lives and scientists do not know very much about them [1].

- Figure 1 - Photos of Sowerby’s beaked whales in the wild.
- (A) A male with prominent teeth. (B) A female and calf. (C) A whale leaping from the water (Photo credits: Lisa Steiner at Whale Watch Azores).
What makes beaked whales so mysterious? Most cetaceans are a little mysterious because they live in oceans, and oceans are huge environments that are challenging for people to explore. Even the biggest whales are difficult to find and study. Cetaceans that are familiar to us tend to live in habitats close to shore, where they are easier to find and study, but beaked whales live in very deep water far from shore. Scientists may spend months on a boat looking for beaked whales and never find them. If scientists do find beaked whales, the whales can dive deep under water for 30 min or more, and the scientists may not be able to find them again when the whales finally come back to the surface. This secretive behavior makes it almost impossible for scientists to learn about beaked whales in the wild [2].
For hundreds of years, scientists have known that beaked whales exist—yet we know almost nothing about them because of their mysterious lifestyles. Most of what scientists do know about beaked whales comes from stranded whales. A stranding is when a whale is sick or dead and washes onto shore. We can look at the stranded whale’s body to learn about what species (type) it is, and sometimes we can also learn what it was eating and how old it is. Using information from these strandings, scientists have discovered 24 different species of beaked whales, and we think there might be even more species! But we wanted to learn even more from stranded beaked whales—where they live, where their food comes from, and how much they move around.
Stable Isotopes Are Nature’s Chemistry Clues
Have you ever heard someone say, “you are what you eat”? Well, when it comes to chemistry, this is true! When you eat a carrot, for example, you receive all the chemical elements inside that carrot, and the carrot received those elements from the soil, water, and fertilizer available while it grew. Over time, special versions of elements, called stable isotopes, build up in our tissues from the foods we eat and the water we drink (Figure 2) [3].

- Figure 2 - The stable isotopes in our bodies come from the environment, through the foods we eat and the water we drink.
- Plants gain stable isotopes from the soil, water, and fertilizer where they are grown, and then those stable isotopes are passed on to us when we eat the plants. The ratio of heavy to light stable isotopes is unique in various regions of the world, so scientists can look at these ratios in the tissues of animals and people to see where they have been living and what they have been eating (Image credit: Aaron W. Kirkpatrick).
Isotopes are forms of the same element that differ in the number of neutrons in the nucleus. When comparing two forms of the same element, forms with more neutrons are called heavy isotopes and forms with fewer neutrons are called light isotopes. There are also protons inside the nucleus, and the number of protons does not change between isotopes of the same element. An isotope of an element that has the same number of protons as neutrons is considered the “regular” form of the element. Some isotopes are radioactive, meaning they decay or break down over time, but stable isotopes do not decay. We can use the element carbon as an example. Carbon has 15 isotope forms, but the most common are 12C, 13C, and 14C. The “C” stands for carbon, and the small number before the C tells us the number of neutrons and protons (added together) that the carbon isotope has in its nucleus. All carbon atoms have six protons, so any difference in the isotope number is due to differences in the number of neutrons. 12C is the regular form of carbon, with six protons and six neutrons, while 13C has six protons and seven neutrons, and 14C has six protons and eight neutrons. Of these three carbon isotopes, 12C and 13C are stable, and 14C is radioactive.
Heavy stable isotopes are rarer than light stable isotopes, but they stay in our bodies longer than lighter isotopes do. When our bodies make more tissues, like skin or muscle, both heavy and light isotopes are used to make those tissues. Some of our tissues, like skin, grow rapidly and are replaced every few months, while other tissues, like bone, grow slowly and may take years to be replaced. Scientists can compare the ratio of heavy to light isotopes in tissues to determine where an animal has been living or where its food and water came from. If scientists have multiple tissues from the same animal, such as samples of skin and bone, we can even compare changes over the animal’s life due to the differences in replacement times for the tissues!
Stable Isotopes Reveal Beaked Whale Secrets
We focused our study on one species of beaked whale, Sowerby’s beaked whale. This species was discovered in 1804 by James Sowerby and it lives in the North Atlantic Ocean, but almost nothing else is known about it. We reached out to museums across North America and Europe, asking to take samples from Sowerby’s beaked whale skeletons in their collections. Many museums work with community-based whale-stranding response programs (to get skeletons from stranded whales) and stranding programs sometimes keep pieces of other tissue types, like skin and muscle. We worked with 25 museums to obtain samples from 102 Sowerby’s beaked whales from the east and West Atlantic Ocean (Figure 3A). Most of these samples were bone, but we got muscle and skin samples from 46 whales. Since bone, muscle, and skin tissues grow at different rates, we could compare isotope values across the whales’ lives, from more than 15 years ago (bone tissue), a year ago (muscle tissue), and a few months ago (skin tissue). This was very exciting—we hoped to learn whether the whales change their habitats or foods throughout their lives.
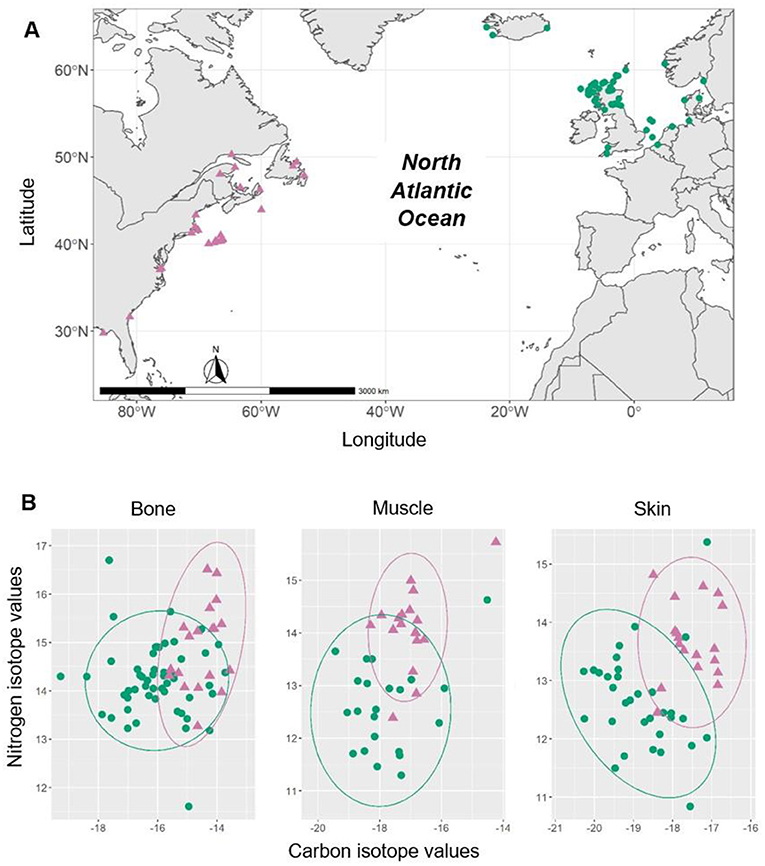
- Figure 3 - (A) A map of the North Atlantic Ocean, showing where the beaked whales in our study were found.
- Pink triangles show whales from the West Atlantic, and green circles show whales from the east Atlantic. (B) Combinations of carbon and nitrogen isotope values for bone, muscle, and skin samples. Circles drawn around the groups of samples, called confidence ellipses, show how similar the combination of isotope values are; if the circles overlap a lot, then the samples have similar values, and if they only overlap a little, then the samples have different values. The circles only partially overlap for all tissue types which, after complicated statistical analyses, tell scientists that the Sowerby’s beaked whales have both short- and long-term site fidelity to the region from which the samples were collected. This species is likely composed of at least two populations whose individuals may interact at some point during their lives.
We analyzed the tissue samples for two stable isotopes: 13C and 15N. 13C is the heavy stable isotope of carbon, and it can help us learn where an animal has been living. 15N is the heavy stable isotope of nitrogen, and it can tell us what foods animals have been eating. When we compared the isotope values between the whales from the east and west Atlantic, we found they were very different (Figure 3B)! This told us that Sowerby’s beaked whales on either side of the Atlantic Ocean have different diets. Next, we wanted to know if whales spend their whole lives in one area, or if they move around a lot. We looked at the isotope values in skin, muscle, and bone tissues from the same whales, and we discovered that the whales were staying in the same place and eating the same foods throughout their lives (Figure 3B). Finally, we asked if we could use the isotope values in a whale’s tissue to determine where the whale lived, and the answer was yes! The isotope values in whales from the east and West Atlantic were so different that we could correctly say where the whale lived based only on its isotope values. Together, these results tell us that Sowerby’s beaked whales live in one area their whole lives. We had discovered something completely new about these mysterious animals!
There is still much to learn about Sowerby’s beaked whales, but now we know that groups of them live in separate habitats and that they do not move back and forth between habitats. This information helps us plan new ways to find and study them so we can learn even more—like the specific foods they eat, if they live in family groups, and how long they live. The information could also help us to conserve these whales. If groups of whales are not moving between habitats, then if one group leaves or dies, beaked whales may be gone from that habitat forever. So, our results are important for people who make policies and rules about ocean noise, pollution, and fishing. Finally, the methods we used can be repeated for other beaked whale species, helping us to learn more about all these mysterious animals. Maybe now, when you think about whales, you will also imagine beaked whales!
Glossary
Cetacean: ↑ The scientific name for the group of animals that contains all whales, dolphins, and porpoises.
Stranding: ↑ When a whale, dolphin, or porpoise becomes stuck in on land; this can happen when an animal is sick or becomes lost and confused.
Species: ↑ A group of the same type of organisms that look similar and can reproduce with each other.
Stable Isotopes: ↑ Isotopes are forms of a chemical element with different numbers of neutrons in the nucleus; stable isotopes do not break down over time.
Neutron: ↑ A tiny, uncharged particle found inside the nucleus of an atom; the number of neutrons in the nucleus can change.
Nucleus: ↑ The center of an atom that contains neutrons and protons; the nucleus is positively charged from the protons.
Proton: ↑ A tiny, positively charged particle found inside the nucleus of an atom; the number of protons in a nucleus is specific to each chemical element type.
Habitat: ↑ The places where animals and plants live; habitats contain all the resources, like food and water, that animals and plants need to survive and reproduce.
Original Source Article
↑Smith, K. J., Trueman, C. N., France, C. A. M., Sparks, J. P., Brownlow, A. C., Dähne, M., et al. 2021. Stable isotope analysis of specimens of opportunity reveals ocean-scale site fidelity in an elusive whale species.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
[1] ↑ Mead, J. G. 2009. “Beaked whales, overview: Ziphiidae,” in Encyclopedia of Marine Mammals (Second Edition), eds W. F. Perrin, B. Würsig, and J. G. M. Thewissen (London: Academic Press), p. 94–7. doi: 10.1016/B978-0-12-373553-9.00027-4
[2] ↑ Madsen, P. T., Aguilar de Soto, N., Tyack, T. L., and Johnson, M. 2014. Beaked whales. Curr. Biol. 24:R728–R730.
[3] ↑ Boecklen, W., Yarnes, C., Cook, B. A., and James, A. C. 2011. On the use of stable isotopes in trophic ecology. Annu. Rev. Ecol. Evol. Syst. 42:411–40. doi: 10.1146/annurev-ecolsys-102209-144726
