Abstract
Silicon is a crucial nutrient that can join with the element oxygen to form a substance commonly called silica. Silica, commonly known as glass, is found in rocks in the Earth’s crust and dissolves into the oceans, where organisms like algae and sponges use it to build their glassy skeletons. This process, called biosilicification, is extremely important in the silica cycle. Over time, organisms have changed the silica cycle. Today, because of these organisms, the oceans no longer contain much silica. However, when the Earth was younger and these organisms had not evolved yet, no biological processes affected silica in the oceans. The evolution of these oceanic organisms across time has removed silica from the oceans. In this article, we discuss how the evolution of silicon-using sponges, as well as tiny organisms called zooplankton and algae, have changed the amount of silica in the world’s oceans through geologic time.
Importance of Silica in the Oceans
Silicon is one of the most common elements on Earth. Silicon can join with the element oxygen to form silicon dioxide, commonly called silica. You probably already know what silica looks like, as it goes by a much more common name—glass. Silica is one of the most common chemicals found in solid rocks and minerals in the Earth’s crust and mantle. Over time, the rocks and minerals on Earth break down into smaller pieces due to wind or rain, in a process called weathering. Eventually, the silica pieces get so small that they dissolve into nearby rivers and streams. From there, the dissolved silica moves into the world’s oceans. Rivers across the globe put a lot of dissolved silica into the oceans every minute of every day. These transformations from solid into liquid form are an important part of the silica cycle, which tracks how silica moves throughout the earth. Dissolved silica in the oceans is an important nutrient for many ocean-dwelling creatures, such as microscopic algae and sponges (Figure 1). These organisms eat up the dissolved silica in seawater to build their skeletons, the same way humans use calcium to build strong bones. A glass skeleton is stronger than you might think, and hard for predators to break, so it is good protection for ocean creatures. The process by which organisms turn dissolved silica in seawater into a glass skeleton is called biosilicification.
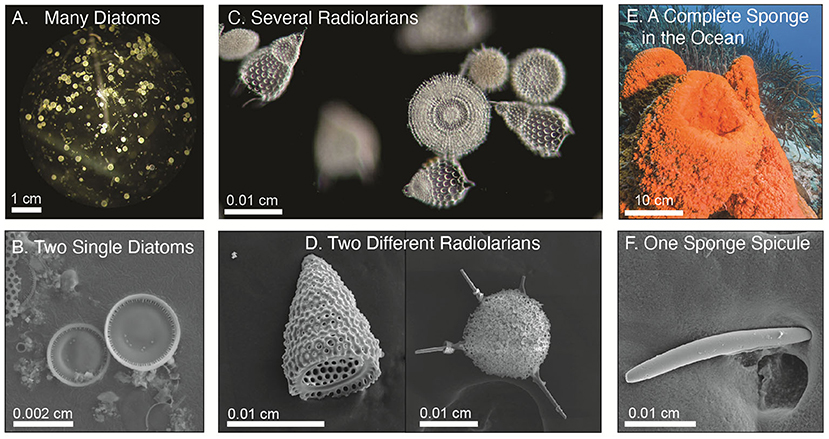
- Figure 1 - What does a glass skeleton look like?
- Several types of ocean organisms have skeletons made of silica, including diatoms, radiolarians, and sponges. (A) A microscopic image of many diatoms. (B) Two single diatoms, close-up. (C, D) Radiolarians. (E) Sponges are much larger, so they can be seen without a microscope. (F) Have you ever wondered what a sponge skeleton looks like? Here is what a piece of sponge skeleton, called a spicule, looks like under a microscope. Scale bars help to show how big each organism is.
Today’s oceans are full of tiny algae made of glass skeletons called diatoms (Figures 1A, B). Diatoms may only be between 0.002 and 0.02 cm in size, but they remove a large amount of the dissolved silica from ocean water to build their skeletons. Diatoms like to stay near the surface of the seawater because, like most plants, they need sunlight to perform photosynthesis and survive. When diatoms perform photosynthesis, they remove carbon dioxide—a greenhouse gas—from our atmosphere. Since diatoms need silica, this means that the amount of carbon dioxide in Earth’s atmosphere is indirectly related to the amount of dissolved silica in seawater.
Without a high-powered microscope, you cannot see an individual diatom, but they are an important food source for animals like zooplankton and fish. Zooplankton are a critical part of the marine food web, as they move energy up the food chain into organisms that larger predators, like humans, like to eat. The flow of energy from phytoplankton to zooplankton to fish is one of the most important processes in the ocean today.
There are also some types of zooplankton, called radiolarians (Figures 1C–E), that like to build their skeletons out of silica. These glass zooplankton are a little bigger than diatoms (between 0.003 and 0.3 cm in size), but you still need a microscope to see them. Finally, some sponges (Figures 1F, G) use dissolved silica in the seawater to build their skeletons out of glass, but they live on the seafloor, far away from the algae and zooplankton hanging out at the ocean surface. Most sponges are only a few centimeters in size, but some can grow up to two meters in length.
These three groups of creatures—diatoms, zooplankton, and sponges—need a lot of silica to build their skeletons. This means that today’s oceans do not have a lot of dissolved silica in them. However, when the Earth was younger, these organisms were not living in the oceans yet, so there was a lot more dissolved silica around. Over time, the evolution of algae, zooplankton, and sponges has removed silica from the oceans. Researchers have only been trying to understand the ocean’s silica cycle for about 34 years [1–3], and in that time a lot of progress has been made toward understanding how silica levels in the world’s oceans got to where they are today [4, 5]. Researchers agree that biosilicification is what has driven the change in the silica cycle over time.
Precambrian Times (540 Million Years and Older)
The Precambrian times cover most of the Earth’s history, from the creation of the planet 4.5 billion years ago to the emergence of complex life forms almost 4 billion years later. During Precambrian times, Earth had little to no life in its oceans (Figure 2). Therefore, the silica cycle was only controlled by forming silica-rich rocks called chert. Chert is a very hard rock that forms when there is so much dissolved silica in the oceans that the silica turns back into a solid form again. Have you ever made rock candy? If you try to dissolve a really large amount of sugar in water, it starts to form hard candy crystals. Similarly, if there is too much dissolved silica in ocean water, it starts to form solid rock crystals. In prehistoric times, people used chert to make arrowheads and hand axes because chert is very hard and sharp when broken. Have you seen a chert tool? Maybe the rock it was made from came from the ocean floor during Precambrian times, when the amount of dissolved silica in the ocean was high because there were no creatures around to eat up all the tasty, dissolved silica floating around.
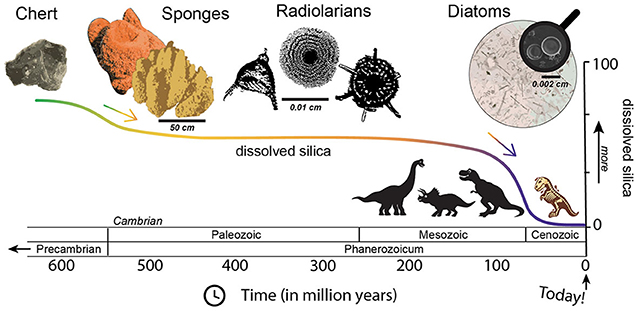
- Figure 2 - Who is eating all the silica?
- The amount of dissolved silica in the oceans has changed since the early Earth. After chert formation and the evolution of sponges and radiolarians, the amount of dissolved silica in the oceans has decreased. Researchers believe that diatoms have been eating most of the silica since the end of the Mesozoic, which is around the same time that dinosaurs went extinct. Scale bars help to show how big each organism is.
Cambrian Times (540 Million Years Ago): Sponges and Zooplankton Arrive on the Scene
You probably think of a sponge as a soft, porous, squishy material used when you are cleaning the house. Sponges are actually animals, and they come in many varieties. Some of them use silica to build their skeletons. Sponges are likely some of the first animals that evolved on Earth. Although not all sponges are still around today, we know that sponges in the Cambrian (at the beginning of the Paleozoic) were the first animals to remove dissolved silica from ocean water to build their skeletons. Researchers observed that, as sponge populations began to increase, the thick layers of silica-rich chert became less common. This tells us that sponges directly impacted the amount of dissolved silica in the oceans.
Just after the appearance of the first sponges, a group of zooplankton called radiolarians appeared as well. While sponges prefer to sit on the ocean floor, radiolarians drift in the seawater. Their populations quickly grew larger, and during the Ordovician (485 million years ago), radiolarians expanded across the globe, resulting in an overall decrease in the amount of dissolved silica in the oceans (Figure 2). Rocks and minerals in the Earth’s crust and mantle continued to dissolve, but organisms were removing a large amount of dissolved silica from the water for the first time. Sponges and radiolarians controlled levels of dissolved silica in the oceans for several million years.
Mesozoic (250 Million Years Ago): Diatoms Take Over!
The Mesozoic was the time when the dinosaurs lived. The Mesozoic includes the Triassic, Jurassic, and Cretaceous Periods—names that might be familiar to you. Did you know that, while dinosaurs roamed the Earth and swam in the seas, something more important was evolving? The Mesozoic saw a huge change in the ocean ecosystem. For the first time, many groups of phytoplankton algae appeared. One group, the diatoms, thrived. As diatoms used silica to build their skeletons, dissolved silica was rapidly reduced in the world’s oceans. Over time, the number of algae in the oceans quickly increased, and dissolved silica was rapidly removed (Figure 2). Algae now controlled the dissolved silica cycle in the oceans and, as their population grew, they kept eating. Diatoms retained control of the silica cycle for the next 200 million years.
Today’s Oceans: Is Any Silica Left Over?
The amount of dissolved silica in the oceans today is still largely controlled by algae. Things like ocean circulation and the newly dissolved silica entering the ocean from rocks and minerals can change on occasion, but overall, once dissolved silica enters the ocean, algae quickly eat it up from the surface waters. Changes in human activity are impacting how much dissolved silica enters the oceans. For example, building dams can reduce dissolved silica input, while eutrophication can impact how much silica is eaten by the algae. Further, as the environment warms, warmer ocean temperatures can affect how many algae grow, which in turn impacts the silica cycle. While there are now more ocean creatures that eat dissolved silica, the three main groups we discussed still largely control the amount of dissolved silica in our oceans. Understanding how these groups evolved over time helps us better understand how our environment will change in the future.
Glossary
Silicon: ↑ The second most common element in the Earth’s crust. Silicon is found in most rocks, sands, clays, and soils. Its atomic number is 14, and the symbol is Si.
Silica: ↑ A chemical compound made up of silicon and oxygen. It occurs in various forms (as in quartz, opal, and sand) and is an important part of glass, concrete, and porcelain.
Silica Cycle: ↑ A cycle that uses biology, geology, and chemistry to track the movement of solid and liquid silica between the Earth’s different systems (like when it moves from the land into the ocean).
Biosilification: ↑ The process by which organisms use dissolved silica to form solid silica, generally to build their skeletons.
Photosynthesis: ↑ A process by which plants/algae produce oxygen and food for themselves and other organisms, using sunlight and carbon dioxide.
Zooplankton: ↑ Small animals in the plankton group. Plankton are aquatic organisms that cannot swim effectively but instead drift with water currents.
Chert: ↑ A sedimentary rock rich in silica. It breaks up into pieces with sharp edges which is why it was used by people to make weapons and tools.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This abstract is part of a series of 6 manuscripts about the marine silicon cycle put together by the ECR SILICAMICS group and validated by Hedwig Ens, Senior Journal Specialist. We thank the SILICAMICS ECRs consortium for its enthusiasm for putting this project together.
References
[1] ↑ Siever, R. 1991. “Silica in the oceans: biological-geochemical interplay,” in Scientists on Gaia, eds S. H. Schneider, and P. J. Boston (Cambridge, MA: MIT Press). p. 287–95.
[2] ↑ Siever, R. 1992. The silica cycle in the Precambrian. Geochim. Cosmochim. Acta 56:3265–72. doi: 10.1016/0016-7037(92)90303-Z
[3] ↑ Maliva, R. G., Knoll, A. H., and Siever, R. 1989. Secular change in chert distribution: a reflection of evolving biological participation in the silica cycle. Palaios 4:519. doi: 10.2307/3514743
[4] ↑ Conley, D. J., Frings, P. J., Fontorbe, G., Clymans, W., Stadmark, J., Hendry, K. R., et al. 2017. Biosilicification drives a decline of dissolved si in the oceans through geologic time. Front. Mar. Sci. 4:397. doi: 10.3389/fmars.2017.00397
[5] ↑ Trower, E. J., Strauss, J. V., Sperling, E. A., and Fischer, W. W. (2021). Isotopic analyses of Ordovician–Silurian siliceous skeletons indicate silica-depleted Paleozoic oceans. Geobiology 19:460–72. doi: 10.1111/gbi.12449