Abstract
A mouthful of water while swimming in a lake is unpleasant but nothing compared to the same situation during a swim in the ocean. A sudden mouthful seawater leaves you gasping for a glass of water to wash the salty taste from your mouth. But have you ever stopped to consider why the sea is salty? In this article, we will dive into the realm of ocean salinity (salt concentration) and show that there is more to it than you may have thought. Where does the ocean’s salt come from? What is it made of and how is salinity measured? Finally, why should the saltiness of the ocean interest us at all?
What is Salt?
Salt is more than just the white crystals in the shaker at the dinner table that we add to food to make it tastier. Let us dig a little deeper. In the ocean, 97% of the salt is made up of ions (electrically charged atoms or molecules) such as sodium (Na+), chloride (Cl-), sulfate () and magnesium (Mg2+) (Figure 1).
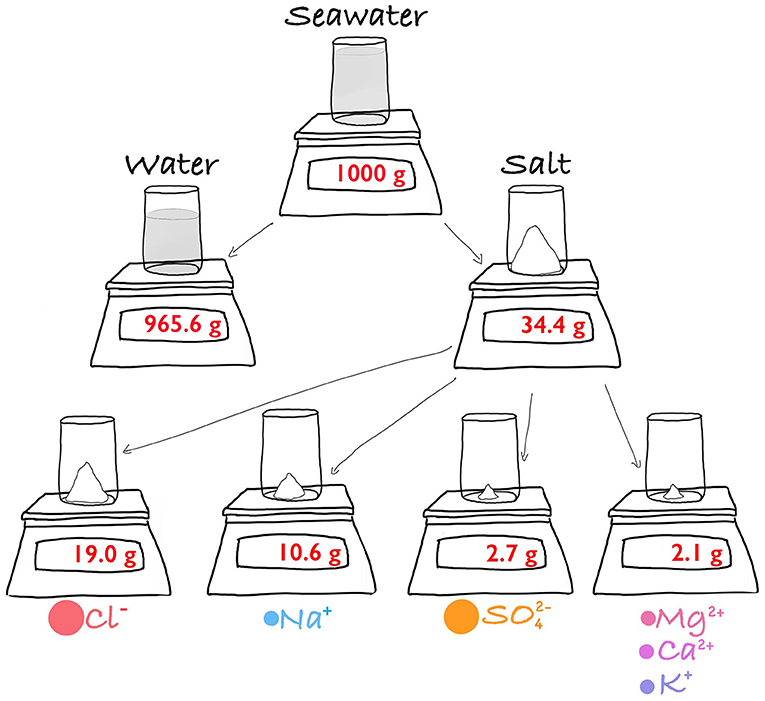
- Figure 1 - What is in a kilo (1,000 g) of seawater?
- Most of it is water (956.6 g). The remainder (just over 34 g) consists of a collection of salts. Chloride (Cl-) and sodium (Na+) are the dominant salts, representing 86% of the total. Much of the remaining salt is sulfate (), Magnesium (Mg2+), Calcium (Ca2+), and potassium (K+).
The ocean receives most of its salt from a process called chemical rock weathering (Figure 2A). The combination of water from rain, plus oxygen (O2) and carbon dioxide (CO2) from the air, acts to react with and dissolve the minerals that rocks are made of. You can see this process in places where rainwater has smoothed rock surfaces, or on statues or stone building decorations that have lost their original shapes.
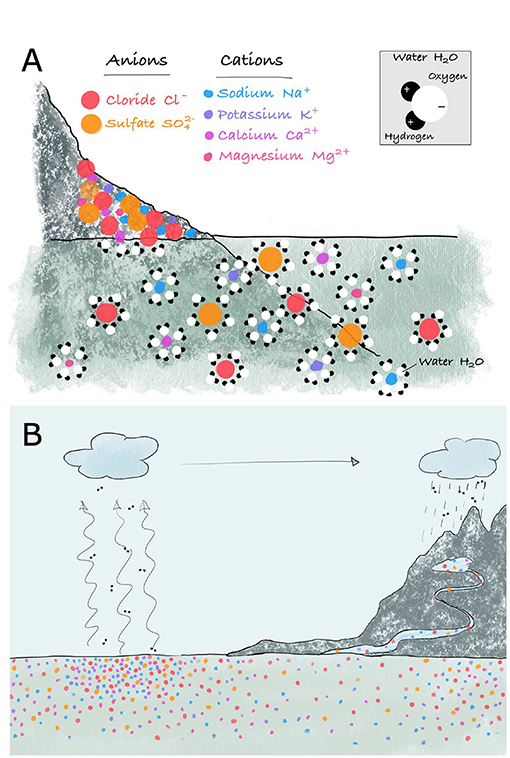
- Figure 2 - (A) Rocks are made of a mixture of anions and cations.
- Water molecules have a weak positive charge at one end and a weak negative charge at the other. Water molecules can surround rock ions, with opposite charges attracting, and isolating the ions from each other. This dissolves the rock, in a process called chemical rock weathering. (B) Salts are eventually carried to the ocean. Ocean salinity is highest in the open ocean, where water is lost to evaporation and the dissolved salts are left behind. Rivers have low salinities, and coastal waters are generally in between.
Water molecules consist of hydrogen and oxygen atoms. The hydrogen end of the water molecule has a slight positive charge, and the oxygen end has a slight negative charge. This makes water an excellent solvent, which means a substance that can dissolve ions. Rocks and minerals contain a mixture of ions, which can be grouped into those with a negative charge (anions) and those with a positive charge (cations). Since opposite charges attract, water molecules surround the ions and isolate them from each other (Figure 2A). So, although river water does not taste salty, it actually does contain salt—just a very low concentration.
Why is Seawater Salty?
Rivers ultimately flow out to the sea, taking the dissolved salts from rock weathering with them (Figure 2B). When ocean water evaporates into the air, the salts are left behind. The evaporated water eventually falls as rain (or snow) over land. This process repeats and supplies more salt to the sea.
But this must only be part of the story, otherwise the oceans would be gradually increasing in salinity, eventually becoming so salty that they could not dissolve any more salt. Seawater is salty, but not that salty! Try experimenting yourself: see how much table salt you can dissolve in 1 L of water. It will be much more than the ~35 g/L there is in the ocean. So, there must be other processes at play that slowly remove salt from the ocean. Oceanographers call these processes a salt “sink,” just as your kitchen sink removes water that comes from the tap.
Salt is slowly removed from the ocean by several processes. Evaporation of water in shallow coastal lagoons can cause the salt concentrations to increase so much that it precipitates and collects on the seafloor. This is how sea salt can be harvested for use in our food. Sea spray can also slowly move salt from the ocean to land. The water in the spray evaporates and leaves the salt behind on land. Finally, saltwater seeping through cracks in the ocean floor near undersea volcanic ridges also slowly remove salt from the ocean. But, on the whole, salt ions linger in the ocean thousands of times longer (several million years) than water molecules do (thousands of years), making seawater saltier than river water.
Measuring Salinity
Worldwide, millions of measurements of ocean salinity are made every day. Let us look at why this is necessary and how it is done. The salinity and temperature of seawater influences the density of seawater. The more salt that is dissolved in water, the denser it is: while 1 L of freshwater at 10°C weighs 1,000 g, 1 L of seawater at the same temperature weighs 1,026 g. Differences in ocean temperature and salinity between depths and locations influence ocean currents (to learn more about this, read this Frontiers for Young Minds article). If we want to understand how the oceans affect local weather, global climate, and the distribution of resources such as fish, we need to understand ocean circulation, and for that salinity plays a role.
Measuring salinity is no easy task. As mentioned earlier, salt is not one substance but a mixture. In the early days of ocean exploration, precise volumes of seawater were evaporated and the salts left behind were weighed. In the 1800s, the Danish geologist Forchhammer went further and determined the concentrations of individual salts [1], which is a very time-consuming process even for just one water sample. After carefully measuring samples sent by explorers from all over the world, Forchhammer discovered that the relative amounts of the various salts in ocean water was almost always the same, which made things much simpler. This meant that scientists could measure just one salt, such as the chloride ion (Cl-), which is present in high concentrations and is easy to measure. Salinity can then be calculated by multiplying by the constant Forchhammer derived: 1.812. This number is remarkably similar to modern estimates (1.815) [2], which is amazing given that he worked with simple equipment and did not even have electric lighting!
In the 1960s, electronic equipment was developed to assess salinity by measuring how well a seawater sample conducts electricity. This is the basis of modern salinity-measuring devices, which can be mounted on drones called Argo floats that are released into the ocean and send data back via satellites (to read more about these, see this Frontiers for Young Minds article). These underwater drones have a collection of sensors that can measure pressure (for depth), temperature, and conductivity (for salinity). They drift with ocean currents and can automatically control how well they float. When they sink and rise, they collect measurements of seawater properties that enable scientists to construct maps of ocean salinity (Figure 3).
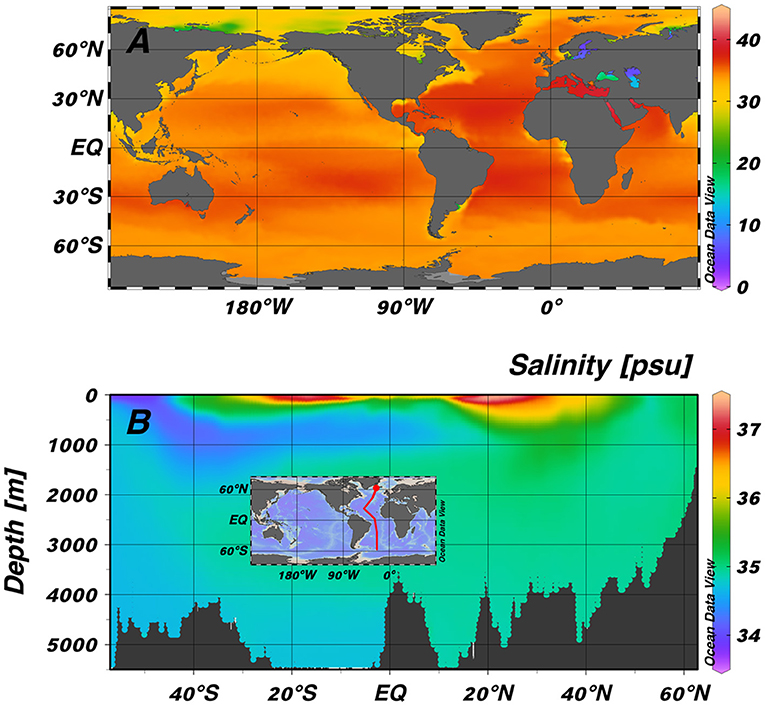
- Figure 3 - (A) The salinity of the surface ocean around the world.
- Dark red colors show the highest salinity, which is often in the tropics where hot weather leads to more evaporation. (B) A slice through the Atlantic Ocean (red line on map), showing how salinity can change with depth. The highest salinities are in surface waters of the tropics (either side of the equator). In deeper waters (below 500 m), the salinity is slightly lower than at the surface (Data from World Ocean Atlas 2018).
The Story Continues
With all this progress, you would think that the puzzle of ocean salinity has been solved. But this is not the case. While Forchhammer’s idea about the constant composition of sea water has been enormously useful, there are actually small but measurable differences in the salt composition across regions of the ocean. Although these differences are small, they are important if we want to accurately describe the properties of seawater. So, scientists are now updating how they calculate ocean salinity, taking into account that the salts in seawater are not so constant after all [3]. After over 150 years, the story continues to unfold. There is more to salt than you might think. Keep that in mind next time a wave takes you by surprise and you get a mouthful of salty water!
Glossary
Ion: ↑ An atom or molecule that has a charge because it has gained or lost electrons. Positively charged ions (Na+) are called cations; negatively charged ions (Cl-) are called anions.
Dissolve: ↑ When individual molecules of a substance become surrounded by molecules of a liquid, such as salt ions in water.
Solvent: ↑ A liquid that can dissolve a solid or gas. Water is an excellent solvent.
Concentration: ↑ The amount of a substance in a volume of liquid or gas; e.g., 34 g of salt in 1 L of water has a salt concentration of 34 g/L.
Evaporation: ↑ When water molecules are warmed up enough that they can move further away from each other, turning a liquid into a gas.
Salinity: ↑ Salt concentration; a measure of how salty seawater is.
Precipitate: ↑ When liquid molecules (such as water) can no longer keep molecules of a substance separate from each other and the substance becomes solid (e.g., salt crystals).
Density: ↑ The mass of a specific volume of gas, liquid or solid. The density of seawater is influenced by water, its temperature and the concentration of substances dissolved in it.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This publication was supported by a grant from the Smed Foundation to AWV and Independent Research Fund Denmark Grant No. 9040-00266B to CS. Figures were designed and created with support from Pernille W. Rasmussen.
References
[1] ↑ Forchammer G. 1865. On the composition of sea-water in the different parts of the ocean. Philos. Transact. R. Soc. London 155:203–62. doi: 10.1098/rstl.1865.0004
[2] ↑ Millero F. J. 2010. History of the equation of state of seawater. Oceanography 23:18–33.
[3] ↑ McDougall, T. J., Jackett, D. R., Millero, F. J., Pawlowicz, R., and Barker, P. M. 2012. A global algorithm for estimating absolute salinity. Ocean Sci. 8:1123–34. doi: 10.5194/os-8-1123-2012