Abstract
The immune system has a complicated job that involves being able to tell the difference between potentially harmful microbes and those that are helpful to humans. Correct immune responses are critical to the maintenance of health, yet sometimes can get out of control and be the cause of disease. Many of our insights into the ways that the immune system works have come recently, but the field of immunology can trace its roots back to the pioneering work of Dr. Edward Jenner and his efforts to vaccinate people against smallpox. This article will serve as the introduction to a multi-part series aimed at understanding the very complex and important topic of how the immune system is able to detect threats, mount appropriate responses, and yet not get out of control. This first article introduces the concept of harmful versus beneficial microbes, how vaccines have impacted health and our understanding of immunology, and the central role of T helper lymphocytes in directing appropriate immune responses.
What You Cannot See Can Hurt You … But Usually Does Not
We live in a world filled with creatures too small to be seen without a microscope. These “microbes” include viruses, bacteria, fungi, and parasites that like to live on or in humans. Most of these microbes are harmless and actually do things that help us. For instance, bacteria in the gut help to break down food into usable parts that we otherwise would not be able to digest. Living with microbes is not only normal but also an important part of being healthy. Cells of the skin, cells lining the airways, and cells making up the walls of the intestines create protective barriers to keep microbes outside of our bodies. However, if the microbes get through these barriers, they can flourish and grow, and even harmless microbes can cause dangerous infections. That is why it is important to wash cuts and keep them clean until they heal. There are a few types of microbes that have developed ways of getting through the barriers in humans, even when there are no cuts. The fact that cuts are part of normal life and that there are some microbes that can enter the body even when there are no cuts make it necessary for us to defend our bodies. This defense against microbes is the very important job of the immune system.
Is it a Friend or a Foe?
At its most basic level, the immune system is a communication network that consists of signals and detectors to receive those signals. Surfaces of cells of the immune system are covered with receptors that help to detect signals from the environment around them. At any given moment, thousands of pieces of information are being received by a single immune cell. The interpretation of these signals is very critical to how the cell responds. Like the signals your brain receives from sensing the world around you through sight, touch, hearing, or smell, immune cells are sensing their surroundings all the time, and hopefully acting accordingly. Since harmful situations are not common, most of the time the immune system does not need to get activated. Scientists who study the immune system used to think that there was not much signaling going on during these quiet times, but now they understand that there is communication occurring even when there is no danger. However, we know a lot more about the signals that the immune system uses to get activated when there is danger, because it is much easier to measure those signals. These activation signals can come from microbes, other cells of the immune system, or even cells that are not part of the immune system. When a dangerous microbe does enter the body, there are specialized cells called antigen-presenting cells (APCs) whose job it is to detect them and capture them (see Figure 1). Because there are so many different types of microbes and cells present in the body, there are multitudes of signals and a large number of receptors for them. The study of the cells of the immune system and how they communicate is called immunology. While most of what we know about the immune system has been learned in the last 50 years, the field of immunology has roots back several centuries when a method called vaccination was first described.
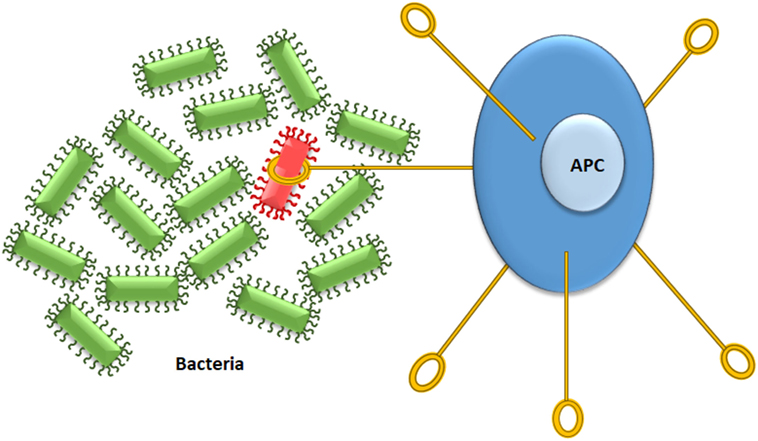
- Figure 1
- Detecting bad microbes is an important job of the immune system. There are specialized cells in the immune system called antigen-presenting cells (APCs) that have the job of identifying potentially harmful microbes and capturing them. The APC then will engulf the microbe, break it up into small pieces, and then display some of these pieces to other immune cells to begin the process of immune activation.
Vaccines have Changed the World
Before vaccines, there were some very deadly microbes that were extremely common in the human population. One of the deadliest of these was a virus called smallpox, named for the small sores that infected people got on their skin. Smallpox was so powerful that people’s immune systems often could not fight it fast enough for them to survive. Whole villages of people could be killed in a short time by the smallpox virus, and in many ways, smallpox shaped much of human history. That began to change in the late 1700s when some very important observations were made by doctors in the English countryside [1]. One thing that they observed was that people who had a minor infection with smallpox and survived seemed to be protected from it afterward. Then, doctors noticed that milkmaids and other people exposed to cows were much less likely to get smallpox than everyone else in town. These doctors began to think that this resistance to smallpox happened because the people in contact with cows were exposed to smallpox-like sores on the cows’ skin during their work, and they often got a mild case of cowpox from those cows. Then, a man named Edward Jenner did a very important experiment, in which he deliberately exposed a young boy to fluid he extracted from a cowpox sore on a milkmaid’s skin. The boy was later protected from getting smallpox. Many more similar experiments became the basis of what we now call vaccination. Despite the facts that no one had ever seen a virus under a microscope (or would for almost 200 more years), and that there was no knowledge of the immune system at the time, this vaccination strategy saved many people’s lives. By 1980, the worldwide effort to vaccinate people to prevent smallpox was so successful that there have been no reported cases of smallpox anywhere in the world since.
Vaccines have now been developed to protect people from other infectious viruses and bacteria. Microbes like polio, diphtheria, and measles, which used to cause very severe illnesses or death, are much less common today because of vaccinations. It took a long time to actually understand why exposing people to cowpox worked to protect people from smallpox. It turned out that the virus that causes cowpox is in the same family of viruses as smallpox. Although it is not common for exposure to one virus to protect people from a different virus, in the case of cowpox and smallpox the viruses were similar enough that the immune response to cowpox was also protective against smallpox. In the case of most other vaccines, it is necessary to either use a killed version of the actual microbe being targeted, or a non-infectious piece of that microbe. Currently, there are very active efforts to develop vaccines against recently discovered microbes such as HIV, Ebola virus and Zika virus. There is also work being done to keep up with rapid changes that other viruses like influenza (flu virus) make to avoid being detected by the immune system.
The Immune System has a Complicated Job
In trying to figure out how cowpox exposure protected people from smallpox, the immune system was discovered. Therefore, Edward Jenner has been credited as being the founder of immunology. It took until the last few decades for immunologists to understand how the immune system responds to viruses or any other type of infections. It turns out that there are very unique types of immune responses that get activated when different microbes infect us, and it is important for the immune system to activate the correct response in each situation. For this reason, the immune system has many branches, each of which has sets of cells specialized to deal with a specific type of infection.
For example, viruses are very small and do not carry with them all of the materials necessary to reproduce themselves. So, when a virus enters a human body, it needs to find a human cell that it can infect. Then the virus needs to take over some of the machinery of the cell to replicate itself and spread viruses to other cells. Every disease-causing virus that you have heard of has mastered taking over human cells in this way. The method by which the immune system normally handles a virus infection is to kill the infected cell before the virus can reproduce itself. It is a race in which the virus often has an early advantage, because it takes some time for the immune system to detect the virus and then to get enough immune cells involved to be able to kill all the cells with viruses inside of them. At the same time, it is very important that this potent cell-destruction mechanism does not get out of control and start killing lots of cells that do not have viruses in them. So, not surprisingly, the branch of the immune system that specializes in dealing with viruses needs to be really good at identifying cells that have viruses living in them, and then killing only those infected cells. Stopping this toxic immune response after the virus is cleared from the body is particularly important and is a job handled by a different branch of the immune system. How well the immune system manages this balance—between racing to get ahead of the virus and then calming down afterward—can be the difference between being healthy or sick, and even between life and death. Other types of microbes, such as bacteria, fungi, and parasitic worms, have very different lifestyles and needs. Thus, the type of immune response that is needed to fight these microbes is very different from the anti-viral response. There are specialized types of cells and factors in the immune system that have the job of detecting the kind of microbe causing the infection and then directing the correct responses to fight these microbes. One of the most critical of these specialized cells is known as the T helper lymphocyte (TH cell).
T Helper Lymphocytes: Directors of the Immune System
Lymphocytes are an important kind of immune cells that are produced from stem cells in the liver before birth and in the bone marrow after birth. Immunologists classify lymphocytes into groups based on their functions. TH cells have receptors on their surfaces that are different from cell to cell, allowing for each TH cell to recognize different microbes. These T-cell receptors can distinguish differences between small pieces of the microbes when these pieces are bound to a molecule called the class II major histocompatibility complex (MHC II), which is on the surface of the APC. A good way to understand this is to think about locks and keys, where each T-cell receptor is represented by a different lock, and there is only one or at most a limited number of keys (peptides on MHC II) that can fit those locks (see Figure 2).
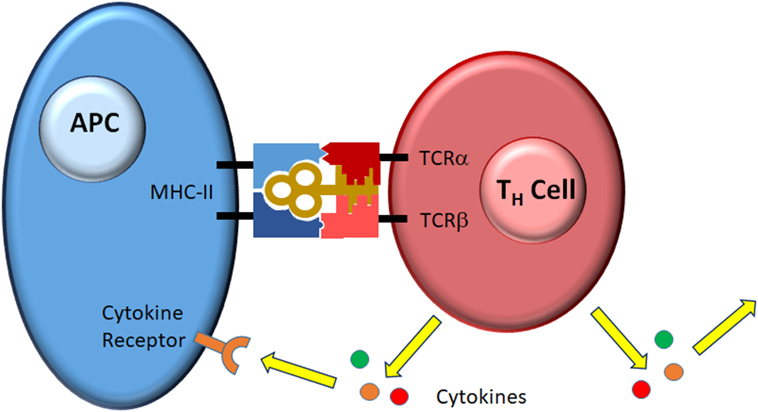
- Figure 2
- A perfect fit. Each T helper lymphocyte (TH cell) has a receptor made up of two subunits, TCRα and TCRβ, that combine to form a unique binding site for antigen bound to class II major histocompatibility complex (MHC II) on the antigen-presenting cell (APC). When the fit is perfect, the TH cell gets activated and senses other signals coming from the APC and the surrounding environment (data not shown). The specific combination of stimuli programs the TH cell to release a set of soluble factors called cytokines that signal back to the APC or travel throughout the body to direct the rest of the immune response. Different sets of cytokines control what type of response develops.
TH cells play a central, essential role in immunity. They are the main communicators in the immune system, and they are highly specialized to interpret the signals that come in from the rest of the body. Correct detection of the amount and type of danger, and communication of that information to other cells of the immune system, are key to any good immune response. When activated, TH cells divide to create more TH cells with the same specificity, and produce soluble factors called cytokines that signal other cells in the body how to fight the infection. When everything goes correctly, the immune response directed by the TH cells leads to clearance of the microbe and elimination of the threat. Then, a second “regulatory” branch of the immune system, driven by different TH cells, has the job of calming down the immune system. After the infection has been eliminated, a small group of the anti-microbe TH cells persists and creates immunologic memory of the microbe. The second time the immune system sees that microbe, it responds more quickly and effectively. It was the development of immunologic memory toward cowpox, and the lucky fact that, to the immune system, cowpox looks very similar to smallpox, that made Edward Jenner’s vaccination work. Millions of lives have been saved by this scientific breakthrough.
Much More to Learn
Immunologists are making new discoveries every day. We now know the immune system is always active, most of the time just collecting information and making sure that nothing bad is happening. Things can go wrong with the immune system, leading to either too much destructive activity, resulting in conditions like allergies or autoimmune diseases, or too much suppression of the immune response, which can lead to chronic infections or poor control of cancer. A better understanding of how the immune system works and how to control it has recently led to very effective treatments for many human diseases. Future articles in this series will explore in more detail how different branches of the immune system operate, and what happens when they do not work properly.
Glossary
Microbes: ↑ Microscopic organisms, including viruses, bacteria, fungi, and parasites, that live in almost every environment, including on and within the human body.
Immune System: ↑ A complex network of cells and organs dedicated to protecting the body from harmful microbes while allowing harmless microbes to survive. The immune system is also involved in repairing damage from other sources such as ultraviolet light, toxins, and sharp objects.
Receptors: ↑ Molecules on the surface of cells that detect the presence of microbes and other signals from the environment and transmit signals that change the behavior of the cell.
Antigen-Presenting Cells: ↑ Specialized cells of the immune system that detect the presence of harmful microbes, capture and digest them, and then present small pieces of the microbe to T helper lymphocytes.
Vaccination: ↑ A method of training the immune system to respond more quickly to a harmful microbe by exposing it to a less harmful version or non-infectious product of the microbe.
T Helper Lymphocyte: ↑ A cell of the immune system that specializes in interpreting the type and level of danger and coordinates the rest of the immune response by sending signals to other cells.
Immunologic Memory: ↑ A quicker immune response that occurs the second time an animal is infected with the same microbe, protecting the animal from reinfection.
Conflict of Interest Statement
The author declares that there are no commercial or financial relationships that create a conflict of interest.
Additional Reading in Frontiers for Young Minds
↑ Flores-Valdez, M. 2016. Why is it important to improve vaccines against latent tuberculosis? Front. Young Minds 4:19. doi:10.3389/frym.2016.00019
↑ Tregoning, J. 2017. Flu, flu vaccines, and why we need to do better. Front. Young Minds 5:7. doi:10.3389/frym.2017.00007
↑ Davis, R., and Hollis, T. 2016. Autoimmunity: why the body attacks itself. Front. Young Minds 4:23. doi:10.3389/frym.2016.00023
↑ Tunnessen, N., and Hsieh, M. 2018. Eating worms to treat autoimmune diseases? Front. Young Minds 6:32. doi:10.3389/frym.2018.00032
Reference
[1] ↑ Brink, S. 2018. What’s the Real Story About the Milkmaid and the Smallpox Vaccine? National Public Radio (U.S.). Available at: https://www.npr.org/sections/goatsandsoda/2018/02/01/582370199/whats-the-real-story-about-the-milkmaid-and-the-smallpox-vaccine (Accessed: July 1, 2018).
