Abstract
In the fight against global warming, it is vital to find ways of producing energy that do not pollute the environment. One of the best solutions for clean energy production is a solar cell, which uses several types of materials to generate electricity from sunlight. In this article, you will learn what a solar cell is, what it is used for, and how it works. I will also present a recent breakthrough discovery in this field: a new, game-changing material called perovskite. This material not only lowers the cost of solar cells, but lets us use solar cells in ways that were never before possible, such as for solar windows; mobile phone and car chargers, and more. Solar energy is clean and non-polluting source of energy. We also do not need to consider how to transport it from one place to another since the sun is everywhere. Additional important point is that the Sun is here and will stay as long as we are here, therefore we just need to use it for our needs and to generate clean energy. Solar cells are the major clean source of energy exists and today we have to find the way how to use it in for different applications.
What Is a Solar Cell?
A solar cell works based on the photoelectric effect, which happens when light hits a special kind of light-absorbing material, called a semiconductor. As the semiconductor absorbs light, it creates negative charges (electrons) and positive charges (holes). The electrons and holes are attracted to each other, and when they join together, they release energy in the form of light. If we can separate the electrons and holes and keep them from coming together, we can then use them to generate electricity. This is the principle on which the solar cell is based.
The solar cell in Figure 1 is made of a semiconducting material that absorbs light. Above and below the semiconducting material are two additional materials—one that attracts the electrons (electron transport material) and another that attracts the holes (hole transport material). At the bottom of the solar cell is a layer of glass that holds the solar cell together.
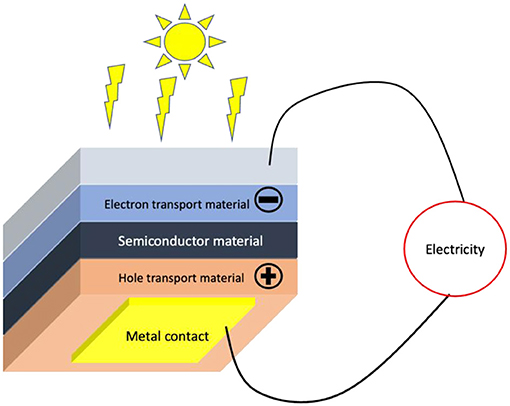
- Figure 1 - How a solar cell works.
- The sunlight comes through the glass into the cell. When the sunlight hits the semiconducting material, it generates electrons and holes which are separated and as a result generate electricity. The wires connected to the solar cell conduct the electrical charges that create the electric current, which can then be used for any purpose.
When sunlight passes through the glass, it hits the layer of semiconducting material, which creates electrons and holes. The electrons and holes are then pulled in opposite directions—the electron transport material pulls the electrons in one direction, and the hole transport material pulls the holes in the other. These charges then flow through electrical wires connected to the solar cell, creating electricity that can be used for any purpose.
What Is Perovskite?
Recently, a semiconducting material called perovskite was discovered. This material works superbly in the solar cell. One of its main advantages is that it is very simple to prepare, which means that solar cells can be made at a relatively low cost compared to the costs of the solar cells that exist today.
Perovskite was originally discovered as a mineral in 1839, by the German mineralogist Gustav Rose. It was named after the Russian mineralogist Lev Perovsky, who discovered its structure. The original perovskite mineral consists of several elements: calcium (Ca), titanium (Ti) and oxygen (O). Perovskite is also known as calcium titanate (CaTiO3).
In its original form, perovskite contains oxygen, which provides him mainly magnetic properties, wide ban gap which are not suitable for use in solar cells. For example, it cannot absorb a wide range of light and does not conduct electricity well in the sunlight. A few years ago, however, researchers made a breakthrough discovery. They prepared a non-oxygen-based perovskite in the laboratory, and they tested it for use in solar cells [2]. This new perovskite could both carry electrical charges in the light and be non-conductive in the dark. The new material could also absorb a wide range of wavelengths. These traits are very well-suited for solar cells.
Why Is Perovskite Special?
Since the discovery of the new form of perovskite, scientists have learned many more interesting things about it. For example, it can absorb specific wavelengths of light by changing its chemistry [3]. How could we use this to our benefit? If we know which wavelength of light we want our material to absorb, we could design the solar cell so that one part of it absorbs light and one part lets light pass through. Such a material could be used to create solar windows, which would be semi-transparent, like regular windows, and generate electricity like a solar cell. This is just one example of the ways that perovskite can be used in solar cells.
Another interesting thing about perovskite is that it can be prepared at low temperatures—about 80°C vs. about hundreds of degrees for today’s solar cells. This is extremely important because a low temperature lowers the cost of production considerably. Also, the ability to prepare a solar cell at a lower temperature makes it possible to build solar cells that are flexible, because flexible substrates can’t hold high temperatures. Flexible solar cells could be used in a variety of fields, such as the automotive industry, agriculture, mobile phones, and more. For example, these solar cells can charge your mobile phone without the need to connect to an external power source.
Building A Unique Perovskite-Based Solar Cell
How this solar cell built? The solar cell is based on oxidized metals, which are TiO2, ZrO2 and ITO. To make this solar cell, as shown in Figure 2, we use nanoparticles of these oxidized metals. Nanoparticles are very small particles at nanometer size. Several layers of these nanoparticles are printed by a printer on a piece of glass (which hold the whole solar cell without this glass the layers can not hold together) and made into a sheet that is porous (full of small holes). This nanoparticle structure is very thin—only a few micrometers in thickness (1 micrometer = 0.000001 meter). The perovskite is then spread onto this thin sheet, and it seeps into all the spaces between the nanoparticles to complete the solar cell.
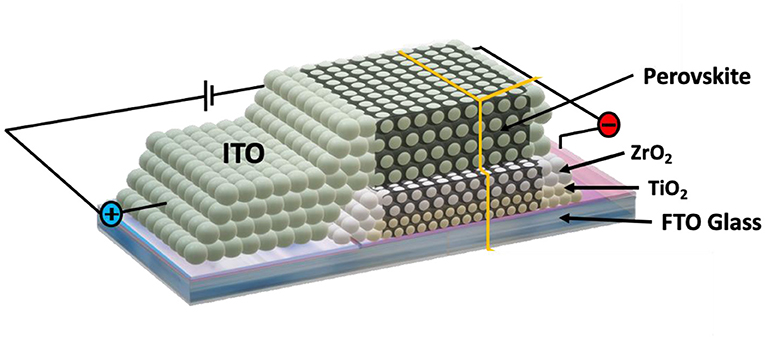
- Figure 2 - The structure of the solar cell.
- The solar cell is made from several layers as mentioned in the figure. [Image credit: [1]].
Testing Perovskite-Based Solar Cells
To measure how well solar cells work, we use a type of lamp that imitates sunlight. The energy range and the spectrum of the lamp is similar to that of sunlight, therefore the lamp simulates the light [4]. During the test, a device called a voltage meter is connected to the solar cell, and it measures the electrical current produced by the cell. With data from the voltage meter, we can create a graphs that describe the efficiency, output, and other characteristics of the solar cell.
An important factor for any new technology—and solar cells in particular—is stability, or the time the solar cell can work without decreasing in its performance. We want to make sure that the solar cells we develop will work for at least 20 years, without any problems. But we cannot wait 20 years to see if they still work! Therefore, we conduct various tests that can tell us how stable the solar cell is and whether it will work for 20 years under severe conditions. In Figure 3, you can see that, in our tests, the solar cells remained stable for over 40 days. This gives us a good indication that these cells will operate for about 20 years without failure. So, among other promising characteristics, perovskite solar cells exhibit good stability.
![Figure 3 - The our perovskite-based solar cells remained stable for over 40 days in our tests, which means they would probably last about 20 years in the real world [Figure credit: [1]].](https://www.frontiersin.org/files/Articles/1091175/frym-11-1091175-HTML/image_m/figure-3.jpg)
- Figure 3 - The our perovskite-based solar cells remained stable for over 40 days in our tests, which means they would probably last about 20 years in the real world [Figure credit: [1]].
- The y axis in all the graphs represents the electrical parameters of the solar cell. Once the values of these parameters are not dropping it means that the solar cell is stable.
Summary
Renewable energies, such as solar energy and wind energy, are increasingly important in the fight against climate change. In the field of solar energy, scientists and engineers have been working hard to develop cheaper, more efficient solar cells. Solar cells containing a mineral substance called perovskite have proven to be very efficient, and testing shows that these cells are likely to keep working for up to 20 years. Perovskite-based solar cell technology has advanced rapidly in just a few years, making this technology a leader among existing solar energy technologies. Researchers are convinced that perovskite technology can be used in many unique new ways, including agriculture and buildings, cars, mobile phones, and more. This specific solar cell technology presented in this work opens the possibility to use solar cells almost everywhere and at any time due to its simple fabrication techniques, low cost, and versatility of the perovskite.
Glossary
Solar Cell: ↑ Generate electricity from sunlight/light.
Photoelectric Effect: ↑ The process by which the impact of light energy creates positive and negative charges in a material, which can be separated and used to generate electricity.
Semiconductor: ↑ A light-sensitive material that conducts electricity when it is in the light and acts as an insulator (not conducting) in the dark.
Electron Transport Material: ↑ Material that conducts electrons.
Hole Transport Material: ↑ Material that conducts holes.
Perovskite: ↑ A semiconductor material used in solar cells.
Oxidized Metals: ↑ Metals containing oxygen atoms.
Nanoparticles: ↑ Tiny particles (0.000000001 meter) that, in our case, crystallize into a porous material with thousands of small holes.
Conflict of Interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Original Source Article
↑ Schneider, A., Efrati, A., Alon S., Sohmer, M., and Etgar, L. 2020. Green energy by recoverable tripleoxide mesostructured perovskite photovoltaics. Proc. Natl. Acad. Scie. U. S. A. 117:31010–7. doi: 10.1073/pnas.2013242117
References
[1] ↑ Schneider, A., Efrati, A., Alon, S., Sohmer, M., and Etgar, L. 2020. Green energy by recoverable tripleoxide mesostructured perovskite photovoltaics. Proc. Natl. Acad. Scie. U. S. A. 117:31010–7. doi: 10.1073/pnas.2013242117
[2] ↑ Etgar, L. 2018. The merit of perovskite’s dimensionality; can this replace the 3D halide perovskite? Energy Environ. Sci. 11:234–42. doi: 10.1039/C7EE03397D
[3] ↑ Cohen, B. E., Li, Y., Meng, Q., and Etgar, L. 2019. Dion–Jacobson two-dimensional perovskite solar cells based on benzene dimethanammonium cation. Nano Lett. 19:2588. doi: 10.1021/acs.nanolett.9b00387
[4] ↑ Grancini, G., Roldan-Carmona, C., Zimmermann, I, Mosconi, E., Lee, X., Martineau, D., et al. 2017. One-Year stable perovskite solar cells by 2D/3D interface engineering. Nat. Commun. 8:15684. doi: 10.1038/ncomms15684
