Abstract
Aging, which is a natural process in which an organism’s functions decline, is present in all living organisms. As we age, our brains begin to break down—certain brain regions get smaller and certain cell types decrease in number. These changes are similar to those seen in diseases like Alzheimer’s and Parkinson’s diseases. The similarities between aging and these neurodegenerative diseases tells us that the same genes might be affecting both processes. However, it is difficult to get samples of human brains to study aging, so we still do not understand what happens to genes in the various regions of the human brain during the aging process. Through global collaboration, a resource called the mouse brain Allen Brain Atlas (BrainMap) of 7 different cell types from cortex has been used, which is like a catalog of genes that are active in the human brain. We downloaded this database as used it as a comparative platform to our brain expression data. The study described in this article shows how protein coding regions of genes involved in different pathways are expressed across the brain, including in specific brain regions and specifically examining different cell type marker genes.
The Human Brain
The human brain is an extremely complex organ consisting of distinct areas that perform various functions. We studied 10 brain regions (see Figure 1).
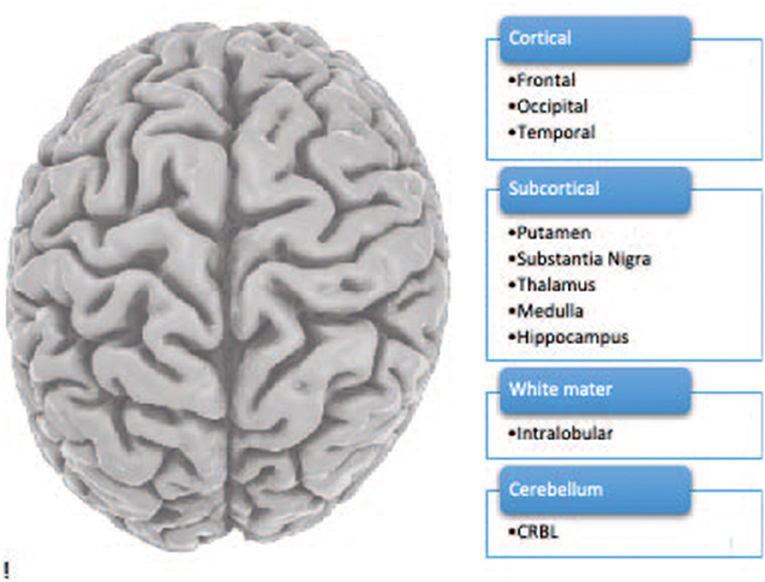
- Figure 1 - The human brain including 10 brain regions.
Like all our organs, at its basic level, the brain is made up of cells. There are actually several types of cells in the brain. The diversity of cell types in the brain is what makes the brain so complex. We studied 7 cell types by stainig and machine learning quantification (see Figure 2).
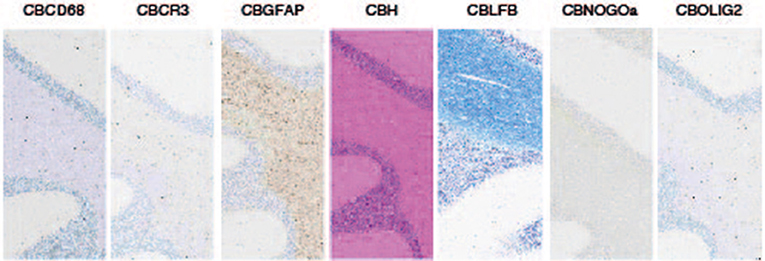
- Figure 2 - The seven stained cell types.
Aging Changes Bodies and Brains
Aging is a natural and unavoidable process. As we age, physical changes happen to our bodies that slowly affect our bodily functions. In short, aging is the process of our bodies breaking down. Some age-related changes are easy to see, such as in microglia and neuronal genes (e.g., TREM1, NEUN). Other changes occur inside our bodies as internal organs and structures begin to break down, such as liver and spleen. Additionally, our telomeres typically gets shorter during aging.
Aging affects the brain, too. It is known that, as we age, the brain decreases in size. Astrocytes and neuronal cell numbers appears as generally preserved in human brains. Microglia showed increase in expression level and oligodendrocytes decrease. We studied RNA expression pattern in 10 different brain regions (temporal cortex, occipital cortex thalamus cerebellum white matter, hippocampus, substantia nigra, frontal cortex, putamen and medulla. We looked in seven cell types (e.g., microglia astrocytes oligodendrocytes neurons oligodendrocyte precursors and endothelial cells).
Certain age-related diseases, like Alzheimer’s disease and Parkinson’s diseases, cause damage to the brain that results in symptoms including motor and cognitive symptoms (e.g., memory deficits, tremor) [1, 2]. Alzheimer’s and Parkinson’s are called neurodegenerative diseases because they cause degeneration (breakdown) of neurons in the brain. Research has shown that, in AD, changes to cells in the brain begin decades before symptoms appear. These changes primarily include decreases in astrocytes, microglia, and endothelial cells. We used exon microarrays on 1,231 post mortem brain samples from 134 individuals in the ages of 16 to 106 obtained from the UK brain bank (UKBEC). We compared young to middle aged and old brain samples.
Studying Genes in Brain Cell Samples
We were interested in finding out more about what happens in the brain as people age, and in neurodegenerative diseases. This is a difficult topic to study because brain samples from people who have died are not easy to obtain. Through my post-doc at UCL ION, I was lucky to have access to 1,231 human brain samples from 134 deceased individuals, aged 16 to over 100. These samples came from more than 10 different brain regions. I analyzed cells from these brain samples to see which genes were turned on or off with aging [3].
To study which genes were turned on in brain cell samples, we used a technique called exon microarrays which contain exonic probe-sets. By comparing young to middle age and old samples (the data was divided to three age groups), the microarray analysis could tell which genes were predominantly detected as altered in each brain region. Nine genes were found as commonly altered in all the regions including non-coding RNA and microglia marker.
Microarray analysis of the human brain samples generated three major key/interesting/important findings.
First, we found that neurons were mainly down regulated.
Second, we found that microglia were up regulated. Third, oligodendrocytes showed mixed pattern of expression changes.
The age-dependent decrease in expression of oligodendrocyte- and neuron-specific genes aligns with the results of brain cell counts, in which we saw decreased numbers of oligodendrocytes and neurons in the frontal cortex.
Together, all of these findings tell us that as the brain ages, neuronal cells decline in expression while microglial cells remain active. These cellular changes might help to explain some of the changes we see in aging, including in inflammatory markers and might mean that microglia are affected more than neurons.
By studying changes in cell-type-specific genes across multiple brain areas, our current study takes a step toward providing the “big picture” of the molecular and cellular changes in human aging brain.
We found significantly altered genes in the aging human, brain including inflammatory and cell type specific markers.
Our study sheds light on the role of microglia in aging and opens new opportunities for microglia replenishment in the aging brain in the future.
Thus, my data may provide insights into the role of glia in the region-specific cellular changes in the aging brain.
Altogether, the data indicate that the cellular changes during aging involve a dramatic shift in the regional identity of glia.
However, through international joint effort, a complete atlas of the brain’s transcription based on samples from two people (the Allen Brain Atlas) has been achieved.
Studies in Mice
We also analyzed RNA-Seq data from microglia-depleted and repopulated young and old mice, simply to study comparative changes to our human brain transcriptome study [4]. Through computational analyses (namely, ANOVA, hierarchical classification) we have found that older mice had lower levels of gene expression for genes from astrocytes, microglia, and endothelial cells, meaning that these cells decreased in the brains of mice as the mice aged. The hippocampus and substantia nigra are the brain areas affected in the early stages of Alzheimer’s disease, and these are the two brain regions where we saw major shifts in the expression of astrocyte and oligodendrocyte-specific genes in older mice. This tells us that parallel expression changes to those seen in the human aging brain can be found in young vs. old mice.
What do These Results Tell us About Alzheimer’s and Parkinson’s?
This study provides a resource for further studies of the relationship between aging and the cellular phase of dementia. It also teaches us on the potential role of microglia in the aging brain. Interestingly, some of the genes that we found to be altered in aging brain were the same genes that were found to be either increasing or decreasing in the blood of Parkinson’s patients [5]. This means that these changes might also be similar. Both decrease and increase of expression changes were detected. Finally, the age-linked changes in the expression of glial-specific genes were most dramatic in the hippocampus and substantia nigra, which are the brain regions affected in Alzheimer’s [6] and Parkinson’s diseases [7, 8].
So far RNA expression changes were hardly studied I aging mainly other pathways were studied e.g., altered intracellular communications genomic instability telomere attrition loss of proteostasis, metabolic dysregulation deregulated nutrient sensing and stem cell exhaustion + caloric restriction (see Figure 3).

- Figure 3 - Main cellular pathways altered in aging.
Conclusions
In this study, we examined brain-wide gene expression patterns in the aging human brain, across a wide age range. We saw increases in expression of microglia-specific genes and decreases in expression of neuron-specific genes. Additionally, we saw major changes in the brain region-specific expression of astrocyte- and oligodendrocyte-specific genes. My study is in particularly exciting since it indicates we can in essence replenish glia in the aging brain (such as done in laboratory mice previously). Also, microglia cell marker genes were enriched in inflammatory functions. Specifically, we showed through t-sne classification analysis clustering of the samples by brain region/age group (by neuronal marker genes), microglia genes lost this classification). We also applied hierarchical classification.
We believe that our data and computational approaches may provide a powerful resource for further study of the cellular and molecular changes taking place during human brain aging and may also provide insights into the pre-clinical cellular phase of dementia.
Glossary
Astrocytes: ↑ A sub-type of glial cells in the central nervous system. They are also known as astrocytic glial cells. Star-shaped, their many processes envelop synapses made by neurons. In humans, a single astrocyte cell can interact with up to 2 million synapses at a time.
Microglia: ↑ A type of neuroglia (glial cell) located throughout the brain and spinal cord. Microglia account for 10–15% of all cells found within the brain. As the resident macrophage cells, they act as the first and main form of active immune defense in the central nervous system (CNS).
Hippocampus: ↑ A complex brain structure embedded deep into temporal lobe. It has a major role in learning and memory. It is a plastic and vulnerable structure that gets damaged by a variety of stimuli. Studies have shown that it also gets affected in a variety of neurological and psychiatric disorders.
The Prefrontal Cortex (PFC): ↑ The prefrontal cortex (PFC) is the cerebral cortex covering the front part of the frontal lobe. This brain region has been implicated in planning complex cognitive behavior, personality expression, decision making, and moderating social behavior.
Transcription: ↑ The first of several steps of DNA based gene expression in which a particular segment of DNA is copied into RNA by the enzyme RNA polymerase.
Conflict of Interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Original Source Article
↑Soreq, L., UK Brain Expression Consortium; North American Brain Expression Consortium, Rose, J., Soreq, E., Hardy, J., Trabzuni, D., et al. 2017. Major shifts in glial regional identity are a transcriptional hallmark of human brain aging. Cell Rep. 18:557–70. doi: 10.1016/j.celrep.2016.12.011
References
[1] ↑ De Strooper, B., and Karran, E. 2016. The cellular phase of Alzheimer’s disease. Cell. 164:603–15. doi: 10.1016/j.cell.2015.12.056
[2] ↑ Dubois, B., Feldman, H. H., Jacova, C., Hampel, H., Molinuevo, J. L., Blennow, K., et al. 2014. Advancing research diagnostic criteria for Alzheimer’s disease: the IWG-2 criteria. Lancet Neurol. 13:614–29. doi: 10.1016/S1474-4422(14)70090-0
[3] ↑ Soreq, L., UK Brain Expression Consortium; North American Brain Expression Consortium., Rose, J., Soreq, E., Hardy, J., Trabzuni, D., et al. 2017. Major shifts in glial regional identity are a transcriptional hallmark of human brain aging. Cell Rep. 18:557–70. doi: 10.1016/j.celrep.2016.12.011
[4] ↑ Elmore, M. R. P., Hohsfield, L. A., Kramar, E. A., Soreq, L., Lee, R. J., Pham, S. T., et al. 2018. Replacement of microglia in the aged brain reverses cognitive, synaptic, and neuronal deficits in mice. Aging Cell. 17:e12832. doi: 10.1111/acel.12832
[5] ↑ Soreq, L., Guffanti, A., Salomonis, N., Simchovitz, A., Israel, Z., Bergman, H., et al. 2014. Long non-coding RNA and alternative splicing modulations in Parkinson’s leukocytes identified by RNA sequencing. PLoS Comput. Biol. 10:e1003517. doi: 10.1371/journal.pcbi.1003517
[6] ↑ Carmona, S., J. Hardy, and R. Guerreiro. 2018. The genetic landscape of Alzheimer disease. Handb. Clin. Neurol., 2018. 148:395–408.
[7] ↑ Soreq, L., Salomonis, N., Israel, Z., Bergman, H., Soreq, H. 2015. Analyzing alternative splicing data of splice junction arrays from Parkinson patients’ leukocytes before and after deep brain stimulation as compared with control donors. Genom. Data, 5:340–3. doi: 10.1016/j.gdata.2015.07.014
[8] ↑ Soreq, L., Salomonis, N., Bronstein, M., Greenberg, D. S., Israel, Z., Bergman, H. 2013. Small RNA sequencing-microarray analyses in Parkinson leukocytes reveal deep brain stimulation-induced splicing changes that classify brain region transcriptomes. Front. Mol. Neurosci., 6:10. doi: 10.3389/fnmol.2013.00010