Abstract
B cells are small white blood cells that contribute to our immune defenses. They produce antibodies, which help us to recover from infections. B cells may cause diseases when they misbehave. If they mutate and then divide out of control, B cells can produce blood cancers called leukemias or tissue cancers called lymphomas. This article traces the development of Rituximab, a drug that kills cancerous B cells. Paradoxically, the drug is itself an antibody—the product of a B cell! Developing such an antibody into a drug required deep knowledge of the immune system, clever genetic engineering, and great effort by scientists and medical doctors. This is a story of several great scientists building on each other’s work to create a drug that has saved many lives.
Introduction
This article tells the story of Rituximab, a drug used to treat cancers of the blood and lymph [1]. These cancers are caused by cells of the immune system that mutate and divide when they should not. Many drugs used to treat such cancers work by killing any cell that divides, including cancer cells, which divide quickly. However, certain normal cells also need to divide all the time, including those lining the gut and the blood-forming cells in the bone marrow. These healthy cells are also killed by the anti-cancer drugs that target dividing cells. The harmful effects of these drugs limit their usefulness.
It would be better to kill only the cancer-causing cells, using a “magic bullet” (scientists use this expression to describe drugs that work very precisely on the cause of a disease, with minimal side effects). Rituximab is a drug that gets much closer to this ideal. Rituximab binds to a molecule called CD20, which is on the surface of certain immune cells, called B cells. Binding of Rituximab to B cells allows the removal of abnormal B cells that cause cancers. Rituximab also removes normal B cells, which are part of our immune defenses, but this is not very harmful: other immune cells can still protect us against most infections. Rituximab does not attack other cells in the body, because they do not have the CD20 molecule. Therefore, this treatment is less harmful and more effective than the older drugs.
The development of Rituximab involved many scientists building on each other’s work over decades. They had to discover how B cells work within the immune system, how they cause disease, and how they can be distinguished from other cell types. They then had to use this knowledge to kill misbehaving B cells in human patients.
B Cells in Defense and Disease
We have all experienced feeling ill and tired, with sniffles, cough, or fever for a few days—and then we slowly start feeling better again. This misery can be caused by a common cold virus that enters our lungs and multiplies in our cells. The immune system fights the virus and restores our health. Without the immune response, we would quickly become a breeding ground for viruses and other infections.
The immune system uses white blood cells and proteins in the bloodstream to find and kill infectious agents. Some white blood cells produce symptoms that help to fight infections: inflammation, tiredness, and fever. Other white blood cells and proteins fight infections in the body by identifying each infectious agent by the unique shapes of its molecules (called antigens). Recognition of antigens allows infectious agents to be attacked much more accurately.
B cells are a type of white blood cell that takes part in the immune response [2]. Each B cell has a protein on its surface called an antibody, which differs slightly from the antibodies on other B cells (Figure 1A). During an infection, B cells meet the infectious agent. If the infectious agent has an antigen to which a B cell’s antibody binds, that B cell will divide. This produces more B cells making the same antibody. Some of these cells will release their antibodies, so they can travel in the blood and bind to the infectious agent wherever it may be. The bound antibody targets the infectious agent for destruction by the immune system. Some of the responding B cells can survive for long periods, providing immune memory—a faster, more powerful response—when the same infectious agent enters the body again later in life. The scientist Niels Kaj Jerne discovered this explanation for immune responses [2, 3].
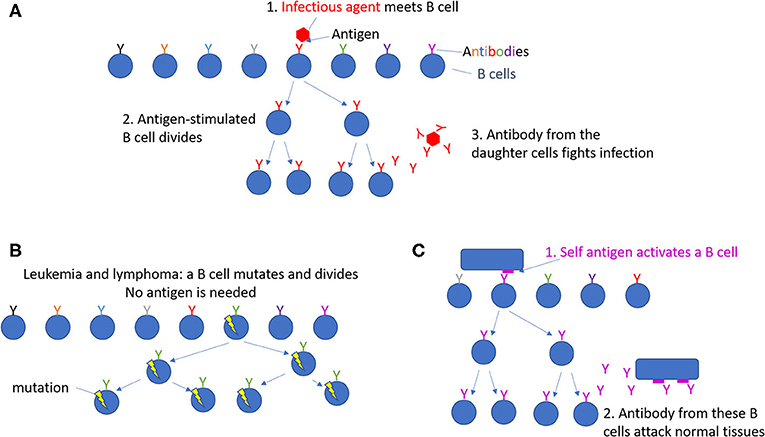
- Figure 1 - Division of B cells.
- (A) Each B cell has a unique antibody on its surface, shown here with various colors. Normal B cells only divide when an infectious agent enters the body. Then, the B cells specific for that infectious agent divide and make antibodies that fight this infection. (B) Leukemias and lymphomas can result from B cells that have mutated so they divide too much, even when no infection is present. (C) In autoimmune diseases, B cells divide and make antibodies that damage normal tissues.
Most other B cells will not participate in this fight, because their antibodies cannot bind the same antigen. They may, however, respond to a different infection, another time. Humans have millions of distinct antibodies on their B cells. Only a tiny proportion of them becomes active in response to any one infection. Together, our B cells can fight any infections we may suffer.
At times, instead of fighting infections, B cells can cause diseases. Sometimes, mutations can occur in a B cell, causing it to start dividing all the time, even though no antigen is present to activate it (Figure 1B) [1]. The mutated B cells build up in the blood as a cancer called leukemia, or elsewhere in the body, forming a cell mass called a lymphoma. The cancerous B cells can disrupt the normal functions of the blood or organs where they build up, ultimately causing death. Many types of leukemia and lymphoma exist; only some come from misbehaving B cells1. In other patients, B cells make antibodies that attack normal, uninfected tissues in the body by mistake (Figure 1C). Diseases caused by such errors are called autoimmune diseases and can attack joints, blood vessels, the brain, or other tissues.
Identifying Human B Cells Using Mice
To develop a drug for treating blood cancers, scientists first had to learn how to distinguish human B cells (including the mutated B cells that form leukemias and lymphomas) from other types of white blood cells. So far, you have learned that antibodies are used by the immune system to recognize infectious organisms…but scientists have learnt how to produce antibodies that recognize specific cell types, like B cells, too! This way of using the immune system was first developed in mice.
In the 1970s, Georges Köhler and César Milstein, working in Cambridge, Britain, used mice to develop a process for creating antibodies against a specific antigen [3]. They started by immunizing a mouse with an antigen. The mouse made an immune response, in which some B cells divided and made antibodies that could bind the antigen. Next, they grew B cells from the mouse one by one in tiny culture dishes and forced them to divide all the time. They were then able to identify, among thousands of such cultures, those B cells that made antibodies specific for the immunizing antigen. Those B cells were grown in large numbers to obtain the unique antibody made by that first B cell and all the cells that grew from it, called daughter cells. A single cell together with all its daughter cells is called a clone, because all the cells make the exact same antibody. The antibody produced by a single clone of B cells is called a monoclonal antibody.
But how did this help scientists identify human B cells? In mice, monoclonal antibodies can be made against any antigen that is foreign to the mice. Since human B cells are foreign to mice, mice immunized with human white blood cells will make some antibodies specific for molecules present on human B cells (Figure 2). Using the method to make monoclonal antibodies described above, scientists identified a molecule called CD20, which was found on human B cells but not on other cells in the human body. When B cells are mutated and become cancerous, they continue to have CD20 on their surfaces. Antibodies that bind to CD20 can therefore be used to identify both normal and cancerous B cells [5].
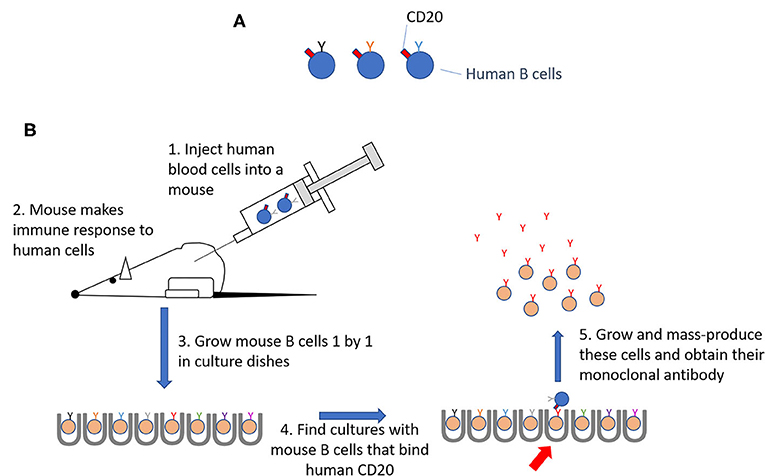
- Figure 2 - Making antibodies that identify human B cells.
- (A) CD20 is on all human B cells. (B) Human blood cells, including B cells, were injected into mice to stimulate an immune response. B cells from the immune mice were grown up one by one. One mouse B cell was found that made an antibody that bound to CD20 on human B cells. This clone was grown as a source of this antibody.
Turning CD20-Specific Antibodies Into a Drug That Works in Humans
We have seen that the immune system kills infectious agents to which an antibody has bound. Is it possible, therefore, to kill B-cell leukemias or lymphomas simply by injecting CD20 antibodies from mice into patients? Unfortunately, antibodies from mice do not work well with the other parts of the human immune system that are needed to kill antibody-coated infectious agents, so one more step was needed to create an effective drug [5].
Antibodies are shaped like the letter “Y” and the different parts of the “Y” have different functions (Figure 3). The antigen binds to the two tips of the “Y,” while the stem of the “Y” activates the immune system to kill. A laboratory process called genetic engineering can be used to create artificial molecules, by arranging genetic material in new combinations. A group of scientists at IDEC Pharmaceuticals Corporation, a biotechnology company in San Diego, United States, did this to create Rituximab [1, 5, 6]. Rituximab contains the tips of the “Y” from a mouse monoclonal antibody that binds to CD20. The remainder of Rituximab comes from a human antibody, which works well in the human immune system. This artificial antibody—part human, part mouse—proved to be an effective drug for killing B cells.
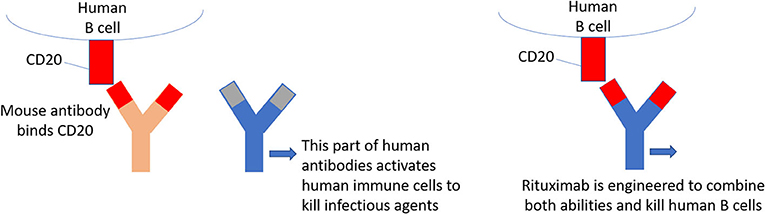
- Figure 3 - How Rituximab was made.
- Rituximab contains part of a monoclonal antibody from a mouse, which binds to human CD20, attached to the rest of an antibody molecule from a human, which can work within the human immune system to kill cells to which the antibody binds. This combination is not found in nature but can be created by genetic engineering.
Rituximab passed safety tests in animals and was then given to patients with B-cell lymphomas. It was a great success, curing more people than a combination of drugs that kill dividing cells, and with fewer toxic side effects. Since 1997 when Rituximab was first given to lymphoma patients on a large scale, patient survival has greatly improved. Combining Rituximab with other drugs was even more effective. Later, benefits were shown in B-cell leukemias [1]. Nowadays, Rituximab is used to treat other diseases in which B cells cause harm without becoming cancerous [7]. We have seen that B cells sometimes cause autoimmune diseases by attacking a normal part of the human body by mistake. In some of these diseases, too, Rituximab, or similar antibodies, have been effective treatments.
Conclusions and Outlook
We have seen that a powerful new drug, Rituximab, was made by first identifying B cells of the human immune system using monoclonal antibodies. Genetic engineering helped to improve the effectiveness of those monoclonal antibodies at killing cancerous B cells in the human body. This story highlights how scientists and medical doctors can work together, over many years, to improve and prolong lives. Rituximab was one of the first monoclonal antibodies developed to treat human blood cancers. Monoclonal antibodies have become important products made by the biotechnology industry for the treatment of many diseases, as well as for diagnosing illnesses and for laboratory research. From the successes (and some failures) of Rituximab and other monoclonal antibodies in the treatment of patients, we continue to learn more about the diseases themselves. Maybe someday you will join us in this effort.
Glossary
Antigen: ↑ The part of an infectious agent that stimulates B cells to make an antibody, and to which that antibody then binds.
Antibody: ↑ A protein on B cells and in blood, which can bind to an antigen. Individual B cells each make a different antibody.
Leukemia: ↑ A disease in which mutated immune cells build up in the blood.
Lymphoma: ↑ A disease in which mutated immune cells build up in solid tissues.
Autoimmune Disease: ↑ A disease in which the immune system attacks a normal part of the body.
Monoclonal Antibody: ↑ An antibody made by a single B cell and its daughter cells, which is produced in large amounts in the lab.
Genetic Engineering: ↑ The laboratory process by which scientists modify DNA to create molecules or organisms with novel properties.
Conflict of Interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
I am grateful to Professor Simon Bowman, Dr. Frances Hall, Dr. Michele Bombardieri, and Dr. Paul Lyons for our collaboration to study T cells in patients treated with Rituximab as part of the TRACTISS trial.
Footnotes
1. ↑ A different paper in this journal [4] describes another type of leukemia and the development of a very different “magic bullet” to treat it.
References
[1] ↑ Borman, S. 2005. The top pharmaceuticals that changed the world: Rituxan. Chem Eng News. 83. doi: 10.1021/cen-v083n025.p110
[2] ↑ Kaiser, G., 2019. Adaptive immunity. In: Microbiology. Unit 6. Available online at: https://bio.libretexts.org/Bookshelves/Microbiology/Book%3A_Microbiology_(Kaiser)/Unit_6%3A_Adaptive_Immunity (accessed August 10, 2020).
[3] ↑ The Nobel Prize in Physiology or Medicine. 1984. Available online at: https://www.nobelprize.org/prizes/medicine/1984/summary/ (accessed August 10, 2020).
[4] ↑ Esposito, M. 2020. Imatinib, the magic bullet for treatment of blood cancer. Front. Young Minds 8:17. doi: 10.3389/frym.2020.00017
[5] ↑ Mohammed, R., Milne, A., Kayani, K., and Ojha, U. 2019. How the discovery of rituximab impacted the treatment of B-cell non-Hodgkin’s lymphomas. J. Blood Med. 10:71–84. doi: 10.2147/JBM.S190784
[6] ↑ Reff, M. E., Carner, K., Chambers, K. S., Chinn, P. C., Leonard, J. E., Raab, R., et al. 1994. Depletion of B cells in vivo by a chimeric mouse human monoclonal antibody to CD20. Blood 83:435–45. doi: 10.1182/blood.V83.2.435.435
[7] ↑ Hofmann, K., Clauder, A.-K., and Manz R. A. 2018. Targeting B cells and plasma cells in autoimmune diseases. Front Immunol 9:835. doi: 10.3389/fimmu.2018.00835