Abstract
Dr. Daniel De Carvalho studies how to fight cancer in new ways. He works in a field called epigenetics, which looks at how cells turn genes on or off. He discovered that cancer cells can be made to look like they are infected with a virus, by turning on old virus-like parts of DNA that are usually kept silent. This process, called viral mimicry, helps the body’s defense system notice the cancer and fight it. Dr. De Carvalho also helped create a blood test that uses epigenetics to find cancer early—even before symptoms appear. This test, which looks at patterns of epigenetic “switches” found on tiny bits of DNA floating in the blood, can sometimes even tell what kind of cancer is growing. His discoveries could help doctors find and treat cancer more effectively, using just a blood sample.
Dr. Daniel De Carvalho was awarded the 2025 Peter Gilgan Canada Gairdner Momentum Award “For the ground-breaking discovery of the role of transposable elements in regulating anti-tumour immunity through viral mimicry, which holds transformative potential for cancer therapy, and for pioneering the development of a novel blood-based test for early cancer detection, classification, and therapy monitoring”.
Cancer: A Hidden Enemy
When you hear the word cancer, you might feel scared or worried. Maybe you know someone who has had it, or you have heard adults talk about how serious it can be. Cancer affects millions of people every year and causes more deaths than almost any other disease. But what exactly is cancer, and why is it so dangerous? And most importantly, how can science help us fight back?
Normally, the cells in our bodies grow and divide in a controlled way, but sometimes, something goes wrong. Cells start growing out of control, ignoring the usual signals to stop. When this happens, a mass of abnormal cells can form, called a tumor. Tumors can start in almost any organ (e.g., lungs, brain, skin, blood) and some can spread throughout the body, taking over organs and blocking the critical functions that keep us alive and healthy.
One thing that makes cancer so dangerous is that the immune system—the body’s natural defense force—does not always catch it. The immune system is great at spotting threats like bacteria and viruses, helping us to recover from common infections. Cancer is more challenging because cancer cells come from our own cells, which means they do not look as “threatening” and can more easily hide from the immune system. Tumors can grow quietly, without being attacked or eliminated, until they become large enough to cause damage or spread.
When Gene Control Goes Wrong
As you probably already know, DNA contains the genetic instructions that tell cells how to build the proteins that help them do their jobs. Some instructions also help control when cells divide or stop dividing. For many years, scientists thought that cancer was entirely caused by changes in the genetic code, called mutations. While mutations that damage the genetic instructions can cause cells to start behaving abnormally and turn into cancer, this is not the whole story. Even when the instructions look normal, problems can still happen—because there is another layer of control involved.
Roughly every cell in the body has the same full set of genetic instructions. For example, skin cells and brain cells contain the same code, but they activate different parts of the code to make only the proteins they need. This system of gene control, called epigenetics, involves chemical tags that attach to DNA and instruct the cell to turn specific genes on or off—like a set of switches. These switches can change over time in response to the environment, diet, stress, or even infection. That flexibility is generally a good thing.
Sometimes, the switches get stuck in the wrong position. Genes that normally protect us from cancer can be turned off, while harmful ones that help cancer to grow stay active. The instructions are undamaged, but the cell no longer reads them correctly. When I learned how epigenetic changes might help explain how cancer works, I became interested in whether we could use epigenetics to fight cancer. But how?
Secrets Hidden in The Dark Genome
As I mentioned, many cancer researchers originally focused on the role of mutations that changed the genetic instructions coding for important proteins. But most of our DNA—an amazing 98%—does not code for proteins. For a long time, this “non-coding” DNA was dismissed as unimportant “junk”, but that view has changed. Scientists now believe that many non-coding regions have important functions. This vast, complex region is often called the dark genome because much of it is still poorly understood.
One specific part of the dark genome particularly caught my attention—stretches of DNA left behind, sometimes by viruses that infected our ancestors thousands or even millions of years ago. These ancient sequences, including a type called transposable elements, are usually silent, kept turned off by epigenetic signals. Transposable elements can copy themselves or move to new spots in the genome, which can sometimes change how nearby genes behave. As I learned more about these transposable elements, I began to wonder: what if we could use epigenetics to turn them back on in cancer cells, to make those cells look like they were infected by a virus? Could that “wake up” the immune system and help it recognize cancer as a threat?
The Virus Alarm Inside our Cells
In healthy cells, the ancient viral sequences are usually kept silent through a common epigenetic “switch” called DNA methylation. This is a normal process in which cells attach small chemical tags called methyl groups to parts of the DNA, to block the activity of instructions that are not needed. My team and I wanted to know what would happen if we turned the quiet transposable elements back on in cancer cells. Might doing so make cancer cells look like more of a threat to the immune system?
When we treated cancer cells in the lab with drugs that remove methylation marks, we saw something exciting: the cells began making a kind of molecule that is usually only produced when cells are infected by a real virus. This warning molecule alerts the immune system that something is wrong. By “waking up” the ancient viral sequences hidden in the dark genome, the drugs caused the cancer cells to act like they were full of viruses. The immune system, which had been ignoring the cancer, started to respond. It was like pulling a fire alarm inside the cell—the warning went out, and the immune system rushed in to fight the cancer cells [1, 2]. We called this effect viral mimicry because it tricks the immune system into seeing cancer cells as if they are infected with a real virus, giving the body a new chance to fight back (Figure 1A).
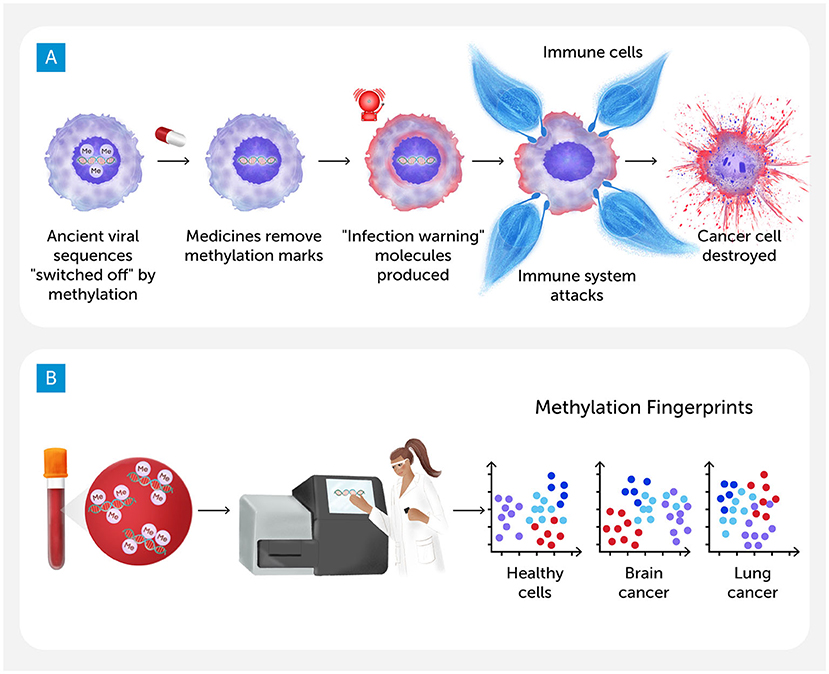
- Figure 1 - (A) We treated cancer cells with drugs that remove methylation marks.
- This “woke up” ancient viral sequences, and the cells produced a warning molecule usually only made when a real virus infects them. The immune system rushed in to fight the cancer cells. This effect is called viral mimicry. (B) Starting with a simple blood sample, scientists can use lab equipment to determine the methylation “fingerprint” on cell-free DNA floating in the blood. These fingerprints are unique enough that we can tell if the cell-free DNA came from healthy cells or cancer cells—and sometimes we can even identify the kind of cancer cells (Figure credit: Somersault18:24).
Finding Better Ways to Spot Cancer
The sooner doctors can detect cancer, the better the chances of treating it successfully. Unfortunately, many people do not know they have cancer until they start to feel sick—by then, it may have already spread, making treatment more difficult. The scans that doctors typically use to try to find cancer can sometimes miss small tumors. If doctors do see something suspicious on a scan, they usually take a small piece of tissue—called a biopsy—to examine further. However, biopsies can be painful or risky, depending on where the tumor is.
What if there was a better way to detect cancer? One promising idea is to use biomarkers—clues in the body that suggest a disease is present. Biomarkers can be proteins or chemicals in the blood, or a pattern in a person’s DNA. My team asked whether epigenetic changes—especially differences in methylation—could work as biomarkers for cancer. Every cell has its own methylation pattern that helps it function normally. In cancer cells, those patterns often change in predictable ways. Some genes are mistakenly turned off, while others are switched on. These changes can be so consistent that they form a kind of fingerprint—a signal that cancer is present, even when we cannot yet see it.
A Drop of Blood—and Lots of Data
The next question was: how can we find each cancer’s unique fingerprint?
As cells get old or damaged, they release tiny pieces of DNA into the bloodstream. These fragments, called cell-free DNA, come from all over the body. In healthy people, most come from normal cells; but in someone with cancer, some come from the tumor. The tricky part is telling the difference.
My team developed an epigenetic method to help solve this problem, called cfMeDIP-seq. It starts with a simple blood test (Figure 1B). We collect cell-free DNA and use advanced tools to scan millions of sites across the DNA, looking for methylation patterns. These patterns help us figure out whether that DNA came from healthy cells or from a tumor. Recognizing the patterns requires powerful computers, which we train to tell the difference between healthy and cancerous DNA. The more examples we give the computer, the better it gets.
cfMeDIP-seq worked better than we expected. In our first studies, we tested blood samples from people already diagnosed with cancer. We could detect cancer-specific methylation patterns even when only a small amount of tumor DNA was present. Eventually, we could even identify what kind of cancer the cell-free DNA came from—such as lung, pancreas, or colon [3]. In another study, we analyzed blood samples from people who were healthy at the time but later developed cancer. In some cases, the test picked up signs of cancer months or even years before symptoms appeared. That means the methylation patterns were already changing long before cancer was diagnosed, giving doctors a chance to act earlier, when treatment is more likely to work. We also used cfMeDIP-seq for brain tumors and found that we could tell the difference between several types of brain tumors without the need for dangerous biopsies [4].
We are still improving this technology, but the goal is clear: to give doctors a faster, easier, and safer way to detect cancer—even before symptoms appear—and to learn important details about each case. This information could one day help guide personalized medicine, allowing doctors to tailor treatments based on the unique features of each person’s cancer—all with just a blood sample.
Curiosity, Challenge, and a Better way Forward
Our work has shown how cancer cells can be unmasked by turning on hidden virus-like parts of the DNA that are usually kept silent. We also found that changes in DNA methylation patterns can help with early detection, using a simple blood test. But I did not start with a grand plan to solve these problems—I followed my curiosity and studied questions that interested me. Over time, those questions led to exciting discoveries, but I did not do it alone. Teams of researchers, doctors, and students worked together across multiple fields, sharing ideas and building tools as a group, and that made all the difference.
When we first started working on viral mimicry, the idea was not widely accepted. It took time—and a lot of evidence—for other scientists to take it seriously. That is starting to change, and the Gairdner award feels like a turning point. At a major genetics conference I recently attended, an entire session was dedicated to viral mimicry—something that would have been unthinkable just a few years ago!
There is still a lot we do not know, but the closer our work gets to real patients—to helping doctors improve peoples’ chances of surviving cancer—the more motivated I become to find the answers. If you are curious about science, my advice is simple: learn to enjoy the journey. The questions you ask, the answers you find—and even the ones you cannot—are all part of what makes science rewarding. What stays with you in the end are the people you help, the people who help you, and the joy of discovery along the way.
Glossary
Epigenetics: ↑ A system that helps cells control which genes are turned on or off, using chemical tags. Epigenetics does not change the DNA itself, but it changes how the DNA is used.
Dark Genome: ↑ The parts of our DNA that do not code for proteins. Once thought to be “junk”, this region includes many elements that help control gene activity or affect how cells behave.
Transposable Elements: ↑ Pieces of DNA that can copy or move to new spots in the genome. Some came from viruses that infected our ancestors. They are usually kept turned off by the cell.
DNA Methylation: ↑ A chemical tag made of a methyl group that attaches to DNA. It helps turn genes off when they are not needed and plays an important role in epigenetic control.
Viral Mimicry: ↑ A process where silent virus-like DNA is turned on in cancer cells, causing them to release signals that alert the immune system, making cancer easier for the body to detect.
Biomarkers: ↑ Measurable signs in the body—like proteins or DNA changes—that show if a person has a disease, how it is progressing, or how they might respond to treatment.
cfMeDIP-seq: ↑ A lab method that uses a blood sample to find cancer by detecting DNA methylation patterns in cell-free DNA, with help from machine learning.
Personalized Medicine: ↑ An approach to healthcare where treatments are chosen based on a person’s unique features, such as their specific cancer type or genetic patterns, to improve results and reduce side effects.
Conflict of Interest
DDDC is cofounder of Adela
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
I wish to thank Dr. Susan Debad for her thoughtful questions, collaborative input, and her contributions as co-author. Figure 1 was created by Somersault18:24.
AI Tool Statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
References
[1] ↑ Roulois, D., Loo Yau, H., Singhania, R., Wang, Y., Danesh, A., Shen, S. Y., et al. 2015. Dna-demethylating agents target colorectal cancer cells by inducing viral mimicry by endogenous transcripts. Cell 162:961–73. doi: 10.1016/j.cell.2015.07.056
[2] ↑ Mehdipour, P., Marhon, S. A., Ettayebi, I., Chakravarthy, A., Hosseini, A., Wang, Y., et al. 2020. Epigenetic therapy induces transcription of inverted SINEs and ADAR1 dependency. Nature 588:169–73. doi: 10.1038/s41586-020-2844-1
[3] ↑ Shen, S. Y., Singhania, R., Fehringer, G., Chakravarthy, A., Roehrl, M. H. A., Chadwick, D., et al. 2018. Sensitive tumour detection and classification using plasma cell-free DNA methylomes. Nature 563:579–83. doi: 10.1038/s41586-018-0703-0
[4] ↑ Nassiri, F., Chakravarthy, A., Feng, S., Shen, S. Y., Nejad, R., Zuccato, J. A., et al. 2020. Detection and discrimination of intracranial tumors using plasma cell-free DNA methylomes. Nat. Med. 26:1044–47. doi: 10.1038/s41591-020-0932-2