Abstract
Problems with the heart and blood vessels, called cardiovascular diseases, are very common—heart attacks, high blood pressure, and strokes are well-known examples. Although medicines and other treatments are available for many cardiovascular diseases, they remain the leading cause of death worldwide. This is because cardiovascular diseases are extremely complicated. They involve multiple body systems and many types of cells, all interacting in complex ways. Also, cardiovascular diseases are slightly different in each patient, which means a “one-size-fits-all” treatment, like a pill to lower cholesterol, does not always work for everyone. Precision medicine is a personalized approach that could help improve cardiovascular disease treatment by collecting loads of detailed data about each patient’s genes, proteins, and cells. Computers and artificial intelligence are then used to figure out the interactions, find patterns, and predict the best treatments. In short, precision cardiovascular medicine could transform the future of cardiovascular disease treatment, improving and saving thousands of lives.
The Global Heart Disease Crisis
The heart is an incredible organ. From before you are born until the end of your life, it never stops working. The heart beats about 100,000 times a day, pumping blood through a vast network of blood vessels that reaches every part of your body. This system—made up of the heart, blood, and blood vessels—is called the cardiovascular system, and it is essential for delivering oxygen and nutrients to cells, removing waste, and keeping the whole body in balance. But when something goes wrong with the cardiovascular system, the consequences can be serious or even deadly.
Cardiovascular disease is the broad name for many conditions that affect the heart and blood vessels. There are too many types of cardiovascular disease to explain them all in this article, but you may already know something about common cardiovascular diseases like heart attacks, strokes, high blood pressure, and irregular heart rhythms. Cardiovascular disease causes more deaths than any other disease. In 2020 alone, about 19 million people worldwide died of cardiovascular disease—this is nearly twice as many deaths as all types of cancer combined [1].
Despite decades of research aiming to understand what causes cardiovascular disease, how to predict who is at the highest risk, and how to treat it effectively, rates of cardiovascular disease keep rising. Why is this happening and how can we fight this growing crisis?
Cardiovascular Disease is Complicated
A major reason why the fight against cardiovascular disease is so difficult is that it is highly variable and does not look the same in every person. Even within the same cardiovascular disease (heart attacks, for example) there is variability in terms of what causes the condition, the symptoms a person has, and how the person reacts to treatments. For example, one heart attack patient might be a smoker with high cholesterol, while another might be a non-smoker with normal cholesterol levels. One patient might experience severe chest pain, while another might feel only mild discomfort or have no warning signs at all. Even at the cellular level, the same disease can take various forms by affecting one biological pathway in some patients and a different pathway in others.
Similarly, common cardiovascular disease medicines work well for some patients but not for others. Drugs called statins, for instance, are widely used to lower cholesterol and reduce the risk of heart attacks and strokes. While statins and other approved therapies work well to reduce bad cholesterol levels, many patients still develop cardiovascular diseases due to other underlying causes such as long-term inflammation [2].
Cardiovascular disease’s variability is shaped by many interacting factors, including a patient’s unique genes, their environment (such as exposure to air pollution, the climate they live in, and access to healthcare), and their lifestyle (diet, exercise, and stress levels) (Figure 1). Variability makes cardiovascular disease extremely difficult to predict, prevent, and treat. Recently, researchers have been shifting toward a new, more personalized approach, which aims to fight cardiovascular disease by considering each person’s unique biology and circumstances. To do so, they must understand the many factors at play—from differences in tiny molecules inside cells to lifestyle patterns across entire populations.
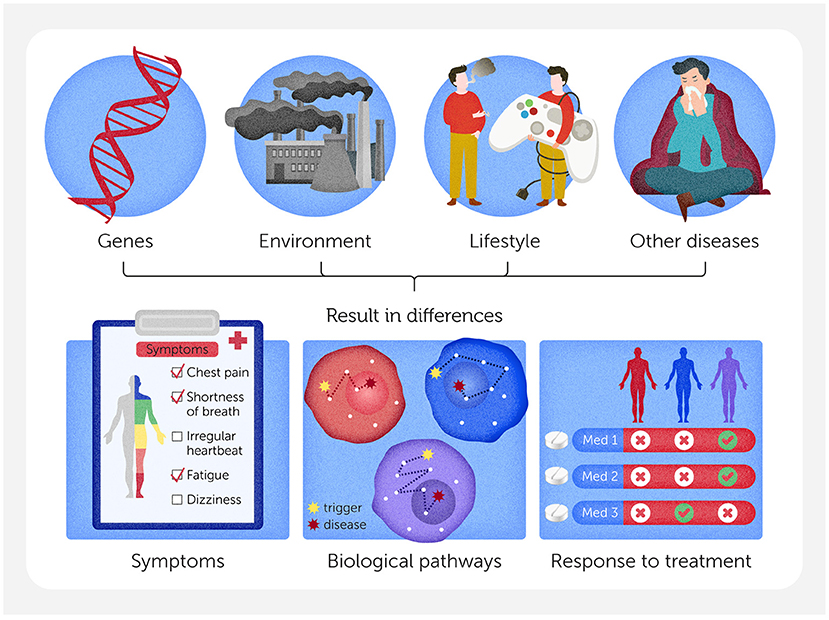
- Figure 1 - There are many reasons why heart disease might not look the same in every person, including differences in genes, environment, lifestyle, or other diseases a patient may have.
- These factors can lead to differences in symptoms, different biological pathways (the step-by-step processes inside cells) that can lead to disease, and differences in the way people with the same disease respond to treatments.
Looking at the “Big Picture” to Understand Cardiovascular Disease
Imagine trying to understand why a power outage is happening in a city by looking at just a single building—it would be impossible to figure out what was causing the problem. But if you zoom out and look at the big picture, including all the neighborhoods, the powerlines that connect them, and the various parts of the power grid that might have failed, you can more easily find the source of the problem and figure out how to fix it.
For years, researchers have primarily used a hypothesis-driven method to study cardiovascular disease. Hypothesis-driven research focuses on a single gene (e.g., ApoE, a gene linked to “bad” cholesterol) or risk factor (e.g., high cholesterol or smoking) and evaluates its contribution to disease. But because cardiovascular disease results from a web of interacting factors, hypothesis-driven research must be supplemented with systems medicine, which looks at the big picture [3]. Instead of looking at one building in a city with no power, a systems medicine approach looks at the entire network of interacting factors. Systems medicine is transforming the way scientists understand complex diseases—it is already being used to study conditions like sepsis, Alzheimer’s disease, and certain types of cancer, for example.
As you can imagine, a systems medicine approach to cardiovascular disease is much more complicated than the traditional method, in which every patient with a certain disease is treated similarly and prescribed the same medicines. So, how do scientists actually study such a complex system?
Decoding Cardiovascular Disease One Cell at a Time
To understand cardiovascular disease and why it is so different from person to person, scientists use an approach called omics. Instead of measuring one thing at a time, omics helps researchers study thousands of molecules, like proteins, at once—giving them a big-picture view of what is happening inside the body. Different types of omics focus on different parts of biology (Figure 2A). By looking at all the proteins at the same time, for example, researchers may see patterns and connections that help them better understand cardiovascular disease, including what makes someone more likely to develop it or how a certain patient’s condition might change over time.
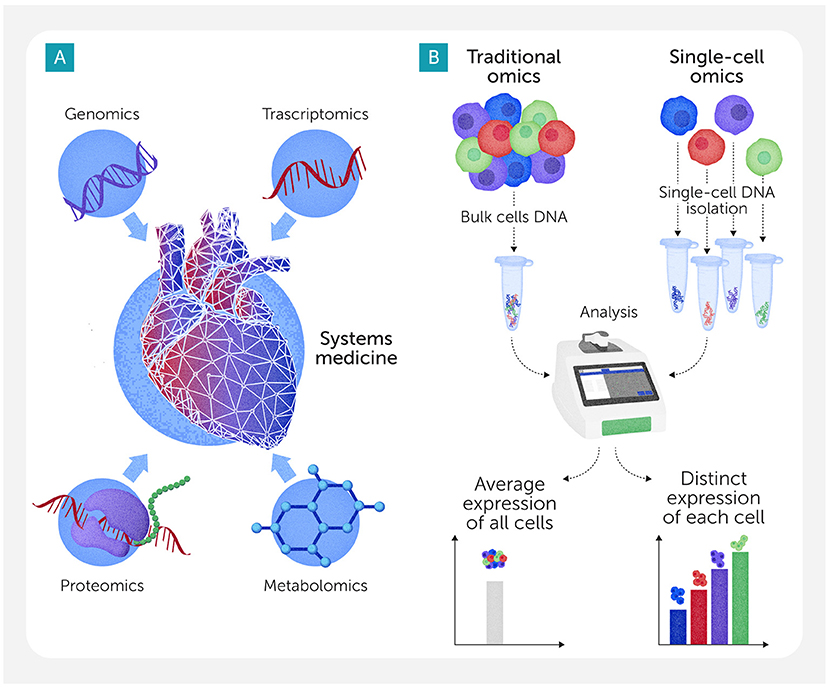
- Figure 2 - (A) Each type of omics focuses on a different aspect of biology.
- For example, genomics looks at which genes are present in an organism’s DNA; transcriptomics reveals which genes are turned on or off in different conditions; proteomics looks at the proteins produced by cells; and metabolomics studies the small chemicals made as cells perform their functions. (B) Traditional omics techniques analyze mixtures of many cells at once, providing an “average” picture of what is happening in a biological sample. By looking at individual cells, single-cell omics can help researchers understand exactly what each cell is doing and how it might contribute to disease.
But there is a challenge: traditional omics methods usually study mixtures of many cells at once, giving researchers only an “average” picture of what is happening. For example, a heart tissue sample can contain muscle cells, immune cells, and cells that line blood vessels, all working together. So, if that sample shows high levels of a molecule, researchers might not know which types of cells are making it, or if some cells are making too much while others are making too little. To solve this problem, scientists have developed single-cell omics, a newer type of omics that studies one cell at a time instead of blending them together (Figure 2B). For instance, single-cell RNA sequencing shows which genes are turned on or off inside individual cells by measuring their RNA. Other single-cell omics methods can study proteins or metabolites in each single cell, similar to how bulk studies characterize them in cell or tissue mixtures.
Understanding these cellular details is essential for pinpointing the root causes of cardiovascular disease. For example, a type of immune cells called macrophages can behave very differently in different people [4, 5]. Some macrophages help heal arteries damaged by cholesterol, while others cause harmful inflammation that makes cardiovascular disease worse. Thanks to single-cell omics, scientists discovered that the balance between such macrophage types in the blood vessels may vary among people. Some types may respond well to cholesterol-lowering drugs like statins, while others do not. This may explain why statins work for some patients but not for others—and could help doctors choose the right treatment more often.
Using AI to Make Sense of Cardiovascular Disease
The powerful tools scientists use to study cardiovascular disease—such as omics and single-cell technologies—produce enormous amounts of data. A single experiment can reveal millions of pieces of information about genes, proteins, or other molecules. Sorting through all that data to find useful patterns would take humans years—if they could do it at all. That is why scientists sometimes use artificial intelligence (AI) to help.
AI is a type of computer technology that can quickly find patterns in “big” data and make predictions based on those findings. In cardiovascular disease research, AI can help scientists figure out which genes or molecules are linked to certain types of cardiovascular disease, and which patterns are connected to higher risk. For example, AI can group patients based on complex patterns in their gene activity, helping researchers discover new subtypes of the disease. These patterns might even be able to predict whether a person’s condition might stay stable or worsen quickly, guiding doctors to choose the best type of treatment for each patient. AI can also look beyond biology. It can analyze other important factors, like exposure to air pollution, diet, stress levels, or access to medical care, to help explain why heart disease develops differently in different people. Using AI, scientists are learning more about cardiovascular disease and moving closer to truly personalized care, including better treatments (If you are interested, this site can help you learn more about AI use in cardiovascular disease).
Better Cardiovascular Disease Medicines
Although many people take medicines to control cardiovascular disease, the number of cases is still rising. The variability we described is one reason—not all medicines work for all patients. But rising case numbers also have to do with the types of medicines that are typically developed. Common cardiovascular disease medicines, like statins to lower cholesterol or beta blockers to control blood pressure, work by blocking or changing the shape of a disease-related protein. Statins, for example, block an enzyme that plays a key role in cholesterol production. But not all disease-related molecules can be treated this way. Some proteins are too large, too flexible, or hidden deep inside cells where they cannot be reached by typical drugs. In other cases, the root of the problem is not a protein at all. This makes it difficult to use traditional medicines to treat all cardiovascular diseases.
To treat cardiovascular diseases caused by these so-called “undruggable targets”, researchers are turning to a new kind of medicine: RNA-based therapies. Instead of targeting proteins after they are made, these treatments work earlier in the process—by controlling how proteins are made in the first place. RNA is a molecule that helps “translate” DNA instructions into proteins within cells. Scientists can design special RNA molecules that stop cells from making harmful proteins or that increase the production of helpful ones (Figure 3A). For example, researchers are testing RNA-based therapies to lower levels of a protein called Lp(a). High levels of Lp(a) contribute to cardiovascular disease, but statins do not significantly lower this protein or may even increase it. So, scientists have developed RNA-based treatments that turn off the production of Lp(a) before it can build up.
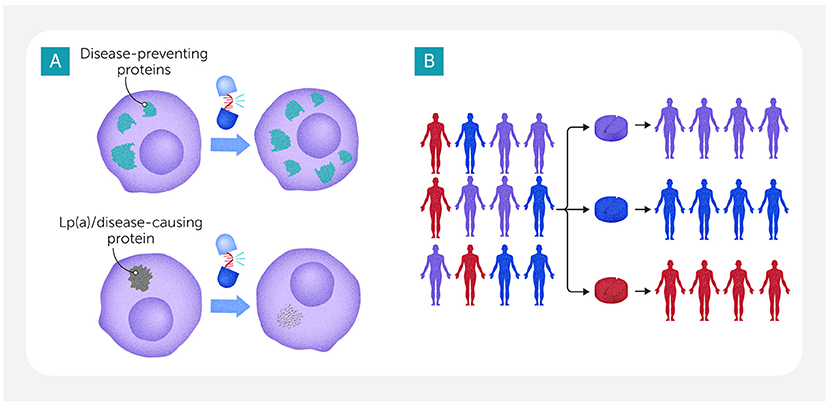
- Figure 3 - (A) RNA-based therapies can help to treat so-called “undruggable” diseases by controlling the way proteins are made.
- These therapies can be designed to help cells make more disease-preventing proteins (top). They can also be created to block or decrease production of a disease-causing protein such as Lp(a), which contributes to cardiovascular disease at high levels (bottom). (B) To improve cardiovascular disease treatment, AI could sort patients into groups based on the results of omics studies or other characteristics, matching each patient with the medicine that will work best for them.
In addition to helping scientists understand cardiovascular disease, AI is also being used to develop better medicines. AI can rapidly scan millions of molecules and predict which ones might work as new drugs. It does this by learning from huge datasets—such as past drug trials or molecular structures—and spotting features linked to success or failure. AI can even design completely new types of medicines that scientists might never have thought to test. As we mentioned earlier, AI can also sort patients into groups based on the results of omics studies or other aspects, helping to match each patient with the best treatment (Figure 3B).
Personalized Cardiovascular Medicine for a Healthier Future
Cardiovascular disease is extremely complex, but powerful new tools like omics, single-cell technologies, and AI are helping scientists understand it better. These tools can reveal why some people get cardiovascular disease while others do not, making it easier for researchers to predict and possibly prevent these dangerous diseases. For a long time, most people with a certain cardiovascular disease were treated the same way, even though their bodies and symptoms could be very different. Now that scientists understand more about how people differ, they can start to match each person with the type of treatment or medicine that works best for their specific biology and situation. For a systems medicine approach to cardiovascular disease to help everyone—not just people who are privileged to have access to the best medical care—scientists, doctors, and policymakers will need to work together on a global level to make sure these advances reach all communities. This kind of borderless cooperation can help build a future where everyone has a better chance at a healthy cardiovascular system.
Glossary
Cardiovascular System: ↑ The body’s system made up of the heart and blood vessels, which works to move blood, oxygen, and nutrients to every part of the body.
Statins: ↑ Medicines that lower cholesterol levels in the blood, helping to prevent heart attacks and strokes by reducing the buildup of harmful fat in blood vessels.
Risk Factor: ↑ Something that increases a person’s chance of developing a disease, like smoking, high blood pressure, or having a family history of heart problems.
Systems Medicine: ↑ An approach to medicine that looks at how different parts of the body—like organs, cells, and genes—interact, using big-picture data to understand disease.
Omics: ↑ A group of tools that study all the genes, proteins, or other types of molecules in a cell or body to understand how it works or what went wrong.
Single-cell RNA Sequencing: ↑ A technique that shows which genes are active in individual cells instead of mixtures, helping scientists understand how different cell types work and how diseases affect them.
Artificial Intelligence: ↑ Computer systems that can learn from data, make decisions, and help scientists find patterns in complex information—faster than humans can.
RNA-based Therapies: ↑ Treatments that use RNA molecules to directly change how cells behave—unlike traditional drugs, which usually work by blocking or activating proteins after they are made.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
MA and SC declared that they were an editorial board member of Frontiers at the time of submission. This had no impact on the peer review process and the final decision.
Acknowledgments
This work was in part supported by the research grants from the National Heart Lung and Blood Institute of the United States (R01HL149302, OT2HL161847, and R01HL 174066 to MA) and Kowa Company, Ltd. (Nagoya, Japan to MA).
AI Tool Statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Original Source Article
↑Aikawa, M., Sonawane, A. R., Chelvanambi, S., Asano, T., Halu, A., Matamalas, J. T., et al. 2025. Precision cardiovascular medicine: shifting the innovation paradigm. Front. Sci. 3:1474469. doi: 10.3389/fsci.2025.1474469
References
[1] ↑ Tsao, C. W., Aday, A. W., Almarzooq, Z. I., Alonso, A., Beaton, A. Z., Bittencourt, M. S., et al. 2022. Heart Disease and Stroke Statistics-2022 update: a report from the American Heart Association. Circulation. 145:e153–e639. doi: 10.1161/CIR.0000000000001052
[2] ↑ Aday, A. W., and Ridker, P. M. 2019. Targeting residual inflammatory risk: a shifting paradigm for atherosclerotic disease. Front. Cardiovasc. Med. 6:16. doi: 10.3389/fcvm.2019.00016
[3] ↑ Loscalzo, J., Barabási, A.-L. s., and Silverman, E. K. 2017. Network Medicine: Complex Systems in Human Disease and Therapeutics. Cambridge, MA: Harvard University Press, p. 436.
[4] ↑ Chelvanambi, S., Decano, J. L., Winkels, H., Giannarelli, C., and Aikawa, M. 2024. Decoding macrophage heterogeneity to unravel vascular inflammation as a path to precision medicine. Arterioscler. Thromb. Vasc. Biol. 44, 2253–2257. doi: 10.1161/ATVBAHA.124.319571
[5] ↑ Decano, J. L., Maiorino, E., Matamalas, J. T., Chelvanambi, S., Tiemeijer, B. M., Yanagihara, Y., et al. 2023. Cellular heterogeneity of activated primary human macrophages and associated drug-gene networks: from biology to precision therapeutics. Circulation. 148:1459–78. doi: 10.1161/CIRCULATIONAHA.123.064794
