Abstract
Organ transplants are operations performed when a critical organ is so sick that it can no longer function to keep the patient alive. About one in every 330 people in the United States has received an organ transplant at some point in their life. Doctors can replace the heart, lungs, liver, kidneys, pancreas, and bowel. Some replacement organs can come from living people, while most come from donors who have died. Once the organ is removed from the donor’s body, it must be kept healthy so that it can work well in its new body. Unfortunately, there are not enough organs available for all the people who need them. Therefore, to save lives, it is important to improve organ donation, preservation, and transplantation. In this article, we describe machines and techniques that can keep organs healthy. Machines can also help doctors decide whether an organ will work well after it is transplanted.
Organ Donation—the Gift of Life
Our bodies are made up of multiple organs that all work together, each doing its own job. Sometimes an organ can get so sick that it cannot get better. In those cases, replacing it with a healthy organ is often the best way to help the person heal. The process in which doctors replace a sick organ with a healthy one is called an organ transplant.
Organ transplantation is fairly common. You might even know someone your age who had a transplant. Take Jamie Fiske, for example [1]. She was less than a year old when doctors gave her a new liver because she was born with a disease that stopped her liver from working properly. Thanks to that transplant, she grew up healthy and strong. Stories like hers show us just how important organ transplants can be. In the United States, about one in every 330 people has received an organ transplant at some point in their life, and other Western countries have similar rates. Today, doctors can transplant the heart, lungs, liver, kidneys, pancreas, and bowel. People who receive an organ transplant often live longer and enjoy a better quality of life [2]. Organ recipients also spend less money on medical care.
Organ transplantation has four main steps (Figure 1). Transplant teams are specially trained to make sure each step happens on time and without problems. It all begins with caring for the organ donor, which means doctors make sure the organs stay healthy by providing the right medicines and careful medical care. The next step is organ retrieval, in which trained surgeons remove the organs from the donor’s body. Next, the organs are stored until they can be transplanted. During this preservation period, the organs need to be kept healthy [3]. Finally, surgeons replace a sick organ with a healthy donor organ during the transplantation surgery.
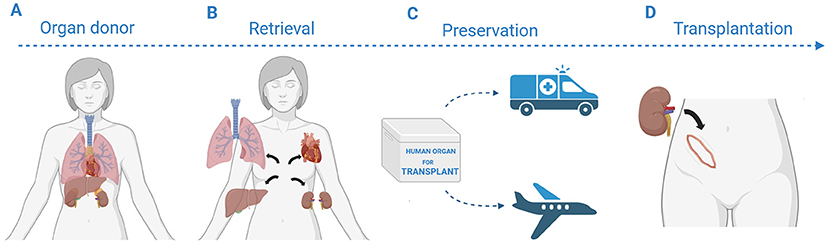
- Figure 1 - The organ transplantation process has four main stages: (A) caring for the organ donor, which means doctors make sure the organs stay healthy by providing the right medicines and careful medical care; (B) retrieval, during which the organ is removed from the donor’s body by trained surgeons; (C) preservation, in which the organ is kept healthy after it has been retrieved; and (D) transplantation, where surgeons replace the recipient’s sick organ with the healthy donor organ.
Organs can come from a living person or from someone who has died. A living donor chooses to donate a part of an organ (e.g., the liver) or a whole organ (e.g., a kidney). A person who has died from a serious injury can also be a donor. That person, or their family, may decide to donate their organs to help someone else live. Even children can be organ donors. For example, Jemima Layzell was only 13 years old when she died of a brain aneurysm, but her organ donation helped eight different people.
Transplantation has helped hundreds of thousands of people survive. Unfortunately, donor organs are not always found on time, and many people die while waiting. Experts estimate that only about 1 in 10 people who need an organ transplant actually get one—so, every donor organ is important! Transplant teams and scientists around the world work hard to help donor organs work better and last longer. In the rest of this article, we focus on how improving organ preservation can help with these goals.
Keeping Organs Healthy Before Transplantation
The time between organ retrieval and transplantation can range from several hours to more than a day. It is very important to keep the donor organs healthy during this time, by making sure the organ’s cells get the energy they need to survive. Normally, energy is made from breaking down the food we eat in the presence of the oxygen we breathe in. In the body, both oxygen and nutrients are transported through the bloodstream. The heart pumps this oxygen- and nutrient-rich blood to every organ, keeping cells alive and active. When an organ is removed from the body, it is disconnected from the blood supply. When cells have used all their saved-up energy, they begin to die. If too many cells die, the organ will be unhealthy and will not work when it is transplanted. So, donor organs need to be kept healthy during the preservation stage.
Lengthening the Preservation Period by Lowering the Temperature
One way to protect an organ during preservation is to slow down its energy use. We can do this by storing the organ at the cold temperature of 4°C. The blood inside the organ is replaced with a cold preservation solution that contains several components: electrolytes which help control the amount of water inside and around cells, energy-providing nutrients like glucose, buffers to keep the organ’s pH at the right level, antioxidants to protect cells from damage, and substances to prevent cells from swelling. Next, the organ is placed in a plastic bag filled with the same cold preservation solution. The bag is stored in a foam box filled with ice. Cold storage is very easy and affordable. Each organ’s structure and function affect how long it can survive outside the body with cold storage. For example, kidneys can usually be kept for about 24 h in cold storage, livers can be preserved for up to 12 h, and hearts can typically last only 4–8 h.
Even though cold storage buys time, the organ is not completely safe. Our cells are not really happy at 4°C, and the longer the organ is stored, the more it is injured. Also, the organ’s cells still use their stored-up energy, slowly but steadily. To help organs work better and longer, researchers are studying how to improve the preservation process.
A Machine to Help the Donor Organs Survive Outside the Body
Organs work together with the rest of the body to stay alive, so how can we keep them working outside the body? Researchers have created machines that do just that! These devices mimic the job of the heart and lungs, so that the organ can keep “living” even outside a person’s body. These machines, called organ perfusion devices, keep blood or a special fluid flowing through the organ (Figure 2) [2]. The perfusion fluid contains sugars, salts, buffers, and preservatives. The organ perfusion device can include a gas exchanger to deliver oxygen (just like the lungs do) and a heat exchanger to control the temperature. By adjusting the temperature, we can keep the organ cold (1–12°C), at body temperature (35.5–37.5°C), or somewhere in between [3].
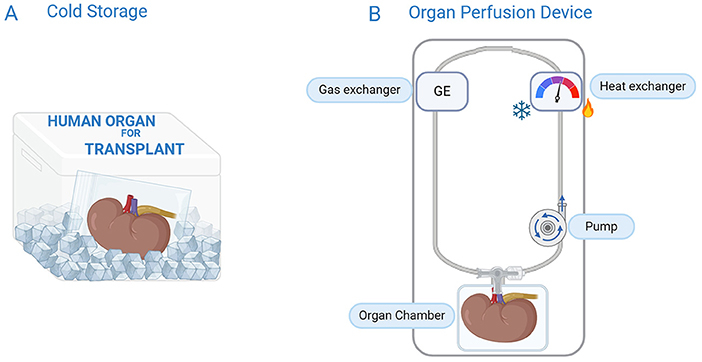
- Figure 2 - Organs can be preserved by cold storage or organ perfusion.
- (A) During cold storage, the blood inside the organ is replaced with a cold preservation solution. The organ is placed in a plastic bag filled with the same cold solution and stored in a foam box filled with ice; (B) During organ perfusion, a machine mimics the body. A pump acts like the heart, delivering a perfusion fluid to the organ. Oxygen can be delivered by a gas exchanger, which acts like the lungs. If a heat exchanger is included, temperature can also be controlled.
Cold Perfusion
Cold perfusion is the easiest organ perfusion strategy. Transplant teams do not need a lot of extra training to use these devices, and the perfusion fluid is relatively simple. Because the organs are cold, the cells work slower and need less energy, and therefore less oxygen. This means we do not need red blood cells to carry oxygen to the cells. Cold perfusion is also a very safe. If something goes wrong with the device, for example if the pump stops working, the organ can still stay healthy as it is kept cold like it would be with cold storage. Cold perfusion has been tested for kidney preservation, and it is clearly better than cold storage [3]. Cold-perfused kidneys work better right after transplantation, and it seems they might function for longer in the recipient. Cold perfusion devices do not need someone to monitor the organ while it is being perfused. This means that transporting the organ to the recipient, even to a far-away hospital, is easy. Initial studies for cold perfusion of livers and hearts are also very promising.
Warm Perfusion
Even though cold perfusion is an upgrade from cold storage, it cannot fully copy the conditions of the body. The low temperature also carries some risk of organ injury. So, warm perfusion is an attractive option, even though it is much more expensive and complex. In warm perfusion, organs are kept at body temperature, so the cells work at full speed and burn a lot of energy. This means the cells need high amounts of oxygen and nutrients, so, the perfusion solution needs to look a lot like blood—with nutrients, red blood cells, and medicines to help the cells survive. The warm perfusion device also needs constant monitoring by a specialist. Initial studies suggest that warm perfusion might be better than cold storage, but this is still being debated among scientists [2–5]. Not all warm perfusion devices are easy to transport, so sometimes the organ is stored on ice first and only placed in the warm perfusion device after it arrives at the hospital where the transplant will take place. This might make the technique less helpful.
“Test-Driving” an Organ Before Transplantation
Donor organs are not always perfect. If the donor died of a serious injury, that event may have also injured the organs—for example through a loss of oxygen and nutrients before organs are retrieved. When injured organs are stored in the cold, the injury worsens. Sometimes a donor organ is so injured that it will not work after transplant, leading to severe medical problems or even death of the recipient. Researchers are therefore looking for ways to "test-drive” organs. We want to predict whether the organ will work in the recipient before we transplant it, by seeing how the organ performs. We can do this during organ perfusion, by checking the organ for signs that it is working well. Researchers already know that cold perfusion is not a good way to test-drive an organ, because the cells are working very slowly. But, during warm perfusion, the organ’s cells are working as fast as they do in the body, so we can check whether the organ is working well before we transplant it [2]. However, researchers still do not fully understand what “working well” looks like when an organ is not connected to the body, but they are learning quickly [2, 5].
Repairing an Organ Before Transplantation
One step beyond “test driving” an organ would be to repair an injured organ before transplantation (Figure 3). We can give medicines during warm perfusion, so perhaps we can discover a medicine that will heal the injury or prevent it from getting worse. We can probably treat bacterial and viral infections during warm perfusion, and we might be able to rebuild parts of an organ or even a whole organ by giving stem cells. Stem cells are special cells that contain the instructions needed to make the cells grow into a specialized type, such as liver cells or heart muscle cells.
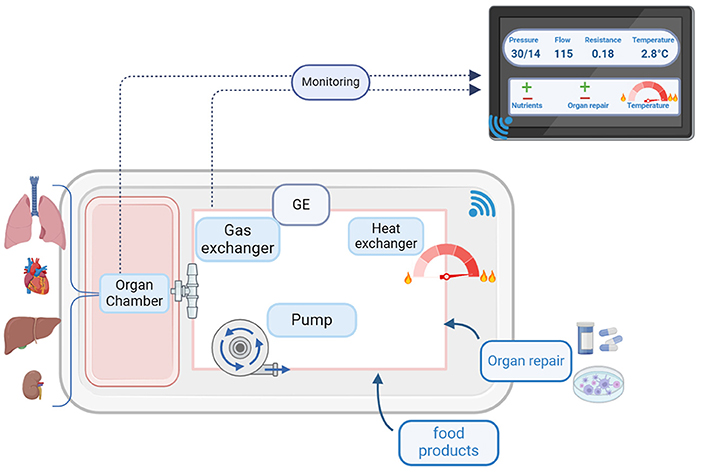
- Figure 3 - Organ perfusion allows us to “test-drive” an organ before it is transplanted, by monitoring the organ to check for signs that it is working properly.
- It may also be possible to repair an injured organ during the perfusion process, by giving it medicine or stem cells.
To Sum Up
Organ transplantation saves lives by replacing sick organs with healthy ones. To keep donated organs healthy until transplantation, transplant teams and scientists are working on methods to preserve them. This is done either through cooling techniques or by using perfusion devices. Researchers are now studying how to improve organ preservation, predict organ function before transplantation, and repair existing injuries. These advances could help to increase the number of transplantable organs, giving every precious donated organ the best chance to thrive in its recipient.
Acknowledgments
This paper was conducted as part of a project supported by funding C2M/23/051 (KU Leuven). We thank Veerle Heedfeld and her daughter Fien Stassen, and Delphine Kumps and her sons Louka and Flinn Dewaet for their helpful suggestions to improve the text toward its target audience.
Glossary
Organ Recipient: ↑ A person who gets a donor organ through organ transplantation.
Organ Donor: ↑ A person who gives an organ to another person who needs it to stay healthy.
Preservation: ↑ Medical efforts and procedures aimed at keeping a donor organ healthy after it has been removed from the donor’s body and before it is transplanted.
Perfusion: ↑ These devices help circulate blood or fluids to organs, often for surgery or organ support.
Gas Exchanger: ↑ This device allows the exchange of oxygen and carbon dioxide, doing the same job our lungs do.
Heat Exchanger: ↑ Device that keeps organs at the right temperature by heating or coolingthem as needed. It is like a thermostat that ensures everything stays at the right temperature.
Cold Perfusion: ↑ A method of preserving organs by pumping cold fluid through them to slow cell activity and reduce energy needs.
Warm Perfusion: ↑ A method of preserving organs by pumping warm, oxygen-rich fluid through them to keep them functioning.
Stem Cells: ↑ Special cells that are able turn and grow into different types of cells because they have the instructions needed to fix and develop new parts or organs.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
[1] ↑ Ettenger, R., Venick, R. S., Gritsch, H. A., Alejos, J. C., Weng, P. L., and Srivastava, R., et al. 2023. Deceased donor organ allocation in pediatric transplantation: a historical narrative. Pediatr. Transplant. 27:e14248. doi: 10.1111/petr.14248
[2] ↑ Verstraeten, L. and Jochmans, I. 2022. Sense and sensibilities of organ perfusion as a kidney and liver viability assessment platform. Transpl. Int. 35:10312. doi: 10.3389/ti.2022.10312
[3] ↑ Tingle, S. J., Thompson, E. R., Figueiredo, R. S., Moir, J. A., Goodfellow, M., and Talbot, D., et al. 2024. Normothermic and hypothermic machine perfusion preservation versus static cold storage for deceased donor kidney transplantation. Cochrane Database Syst. Rev. 2024:CD011671. doi: 10.1002/14651858.CD011671.pub3
[4] ↑ Langmuur, S. J. J., Amesz, J. H., Veen, K. M., Bogers, A. J. J. C., Manintveld, O. C., and Taverne, Y. J. H. J., et al. 2022. Normothermic ex situ heart perfusion with the organ care system for cardiac transplantation: a meta-analysis. Transplantation. 106:1745–53. doi: 10.1097/TP.0000000000004167
[5] ↑ Gilmour, J., Griffiths, C., Pither, T., Scott, W. E., and Fisher, A. J. (. 2020). Normothermic machine perfusion of donor-lungs ex-vivo: promoting clinical adoption. Curr. Opin. Organ Transpl. 25:285–92. doi: 10.1097/MOT.0000000000000765