Abstract
Fungi are tiny organisms found everywhere—in nature, in our homes, and even in and on our bodies. Similar to how people cooperate in cities, fungi can form cooperative communities known as fungal biofilms. Biofilms consist of clusters of fungal cells sticking together, and they can often survive on surfaces for long periods, making them difficult to eliminate. Fungal biofilms are especially dangerous in healthcare settings, because they can grow on surfaces and medical devices. Scientists are actively investigating ways to tackle this issue, such as using combining medicines or using drugs in new ways. In this article, we will talk about how fungal biofilms form, how they can cause problems in hospitals, and how they can be managed. Understanding and addressing these challenges are essential for keeping patients safe from fungal infections.
Living in Communities is not Just for Humans
Imagine living in a big community where everyone works together as a team. In this community, the older members, who have been there the longest and have the most experience, form the foundation of the community. These elders can give rise to new members, helping them settle in and grow within the group. The community also contains younger individuals who think and act quickly, but they still rely on the older ones for guidance. All the group members can collaborate to protect the entire community. This community can also get what it needs from the surrounding environment to survive and grow. Does this sound similar to the lives of communities in our cities? Although it does, we are actually talking about communities of tiny organisms!
What is a Biofilm?
Fungi are microorganisms that can exist either as single cells or as multicellular organisms. When people think of fungi, they often think of the mushrooms that grow in gardens or forests. However, fungi can also live in social communities called biofilms. In fact, they prefer to live together rather than alone, because they can communicate and work with each other to survive better (Figure 1).

- Figure 1 - A biofilm works like a big community, in which cells of different ages and functions work together to help each other survive and eventually spread to other places.
- This can be especially dangerous when biofilms grow on medical devices in hospitals.
In a biofilm community, fungal cells stick to each other and to both living and non-living surfaces. These communities of fungal cells form a protective cover called the extracellular matrix, which acts like a strong shelter. It keeps the cells safe, provides nutrition, and helps the fungi survive. Fungi in biofilms have many advantages over those that live freely. Biofilms are tough to remove from surfaces because the matrix acts like a glue that firmly attaches the fungi to surfaces. Biofilms are a clever survival strategy for fungi because, within them, cells can do specialized jobs and work together to thrive [1].
How are Biofilms Formed?
A biofilm is formed through several steps (Figure 2). During the first stage, fungal cells stick to either living or non-living surfaces, as well as to each other, forming a thin layer of cells. These attached cells collectively produce the extracellular matrix, which is a slimy layer containing sugars, proteins, lipids, DNA, minerals, and water. As we mentioned earlier, the extracellular matrix helps fungal cells to stick to surfaces and it protects them. Once the matrix is formed, more cells can join the community. The fungal cells start growing and make copies of themselves, forming a larger community. The biofilm keeps growing and changing, with some cells becoming specialized in various functions to help the group survive better. Over time, the extracellular matrix solidifies, creating an even more protective structure that shields fungal cells within the biofilm from the surrounding environment [1].
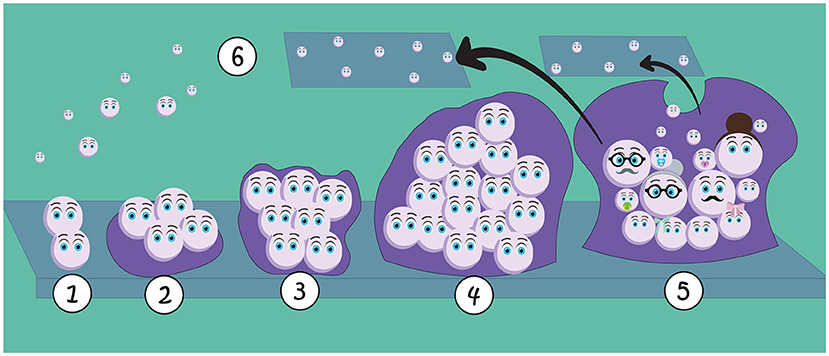
- Figure 2 - Biofilms form through several steps: (1) the first fungal cells stick to a surface and to each other; (2) cells begin to produce the matrix that helps them stick to the surface; (3) the matrix starts protecting fungal cells; (4) new cells join the biofilm, which becomes a larger community; (5) the biofilm keeps growing, with more cells specialized with different functions.
- The final matrix also creates a protective cover, shielding the fungal cells from the environment; (6) Cells can detach from the biofilm and spread into the environment, colonizing new areas.
Cell dispersion is another important event in the biofilm lifecycle. During dispersion, cells detach from the biofilm and spread into the surrounding environment [2]. This allows the fungi to colonize new areas, like seeds starting new biofilm communities in diverse locations, like soil, oceans, plants, our homes, and even our bodies.
Why are Fungal Biofilms Dangerous to Humans?
Many microorganisms, including fungi that cause infections in humans, grow in biofilms. In some situations, fungal biofilms can pose a serious threat to human health. For example, patients in hospitals are often at high risk for infections because their bodies’ defenses may be weakened due to medications, recovering from surgeries, or other medical treatments. Fungal infections associated with biofilms are a huge problem in healthcare all over the world. The health risk varies depending on the type of fungal species in the biofilm, the person’s health, and where the biofilm forms. For example, the fungus Candida auris can create strong biofilms on surfaces in hospitals, like exam tables or surgical instruments, where they can persist for months, even if disinfectants are used to try to kill them (Figure 3B). This persistence helps the fungi to spread among patients. Removing biofilms from hospital surfaces is a big challenge because their matrix is similar to an armor.
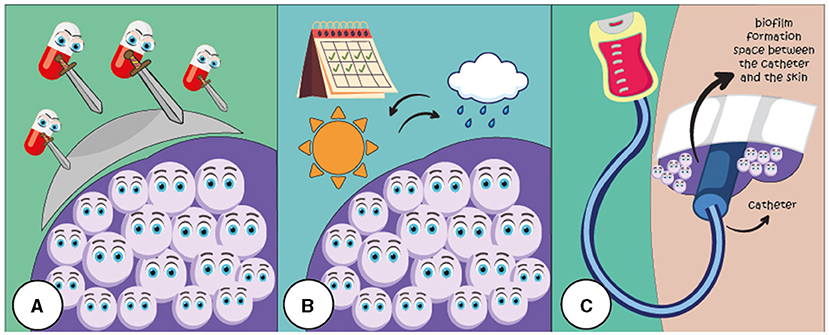
- Figure 3 - Fungal biofilms create several challenges in the hospitals.
- (A) The extracellular matrix can shield fungal cells within the biofilm, blocking antifungal drugs from eliminating the fungi. (B) Biofilms help fungi survive on various surfaces for days or months. (C) Biofilm formation can occur on medical devices like catheters, used to deliver medicines or fluids directly into the patient’s body, which can cause problems for hospitalized patients.
Fungal biofilms can also develop on medical devices like catheters, which are tubes that doctors use to deliver medicines or fluids directly into the body. Treating these infections is difficult because removing the catheters can be very uncomfortable, and patients usually still need them for treatment. This can make infections linked to these devices last longer and return more often (Figure 3C).
If fungal biofilms form in the human body, eliminating them is hard because they often do not respond well to fungus-killing medicines called antifungal drugs. This happens due to the protective extracellular matrix and the ways cells stick together in the biofilm, which blocks antifungal drugs from reaching the fungi. Fungi in biofilms can even become completely drug resistant, making them especially dangerous for patients (Figure 3A).
How can we Fight Fungal Biofilms?
Fungal biofilm infections are usually treated with strong antifungal drugs in high doses. However, giving these drugs in high amounts can be toxic to important organs like the kidneys and liver, causing serious problems. Also, as we mentioned, in many cases these treatments do not work well because the biofilms are resistant to antifungal drugs, making the treatment of such infections even harder [3].
Scientists have been studying new ways to treat infections with fungal biofilms. One effective way is by mixing several antifungal drugs together, or giving them to the patient one after another. This strategy, called combination therapy, can be better than giving drugs individually because the combo attacks different parts of the biofilm all at once, usually giving better results. Combination therapy also allows doctors to use lower doses of each drug, which can reduce the chances of negative side effects for the patient.
Instead of creating new drugs from scratch, another new kind of treatment involves testing out drugs that have been in use for years for other purposes, to see if those drugs can kill fungal biofilms. This approach is called drug repurposing and it can save a lot of time and money compared to making new drugs from scratch. Drug repurposing also saves time because scientists can select drugs that have already undergone safety tests for human use [4].
Another strategy to prevent biofilm formation is to stop the first fungal cells from sticking together in the first place. Special surfaces that prevent fungal cells from sticking can be used to coat medical tools or elsewhere in hospitals to lower the risk of biofilm-related infections. For example, scientists have discovered that electrical currents can reduce biofilms. With this knowledge, they can create surfaces that conduct electricity, to send currents that can “clean” medical devices by killing fungal cells and preventing biofilm formation [5].
Why Fighting Fungal Biofilms Matters?
While we usually think of fungi as mushrooms in natural settings, it is important to know that they can form large social groups called biofilms. Every day, we all come into contact with fungi and their biofilms. These biofilms can be especially dangerous for hospital patients. Combating resistant fungal biofilms often requires several approaches to tackle characteristics like how they form, stick around, and resist drugs. These challenges motivate scientists to study different fungal species and their biofilms, looking for new ways to deal with the infections that they cause. Hopefully this research will help us find better ways to protect patients and prevent fungal infections.
Glossary
Biofilms: ↑ Groups of microorganisms that stick to surfaces and each other, forming a slimy, protective layer that helps them stay together and resist removal.
Extracellular Matrix: ↑ The protective, sticky layer that surrounds and supports cells in a biofilm, helping them stick to surfaces and stay safe while providing nutrients.
Dispersion: ↑ The process by which cells break away from a biofilm and spread out to form new biofilms elsewhere.
Catheters: ↑ Thin, flexible tubes used to deliver medicines or fluids directly into the human body.
Antifungal Drugs: ↑ Medicines used specifically to treat fungal infections.
Drug Resistant: ↑ When certain microorganisms, such as fungi, no longer respond to a drug that is typically effective in eliminating them.
Combination Therapy: ↑ Using two or more different treatments together to improve effectiveness and target a disease more thoroughly than a single treatment alone.
Drug Repurposing: ↑ Finding new uses for existing medicines that were originally developed for different diseases or conditions.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This study was supported by grants from the Brazilian Agencies: Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Fundação de Amparo à Pesquisa no Estado do Rio de Janeiro (FAPERJ), and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). The author(s) declare that financial support was received for the research, authorship, and/or publication of this article.
References
[1] ↑ Le, P. H., Linklater, D. P., Medina, A. A., MacLaughlin, S., Crawford, R. J., and Ivanova, E. P. 2024. Impact of multiscale surface topography characteristics on Candida albicans biofilm formation: from cell repellence to fungicidal activity. Acta Biomaterialia 177:20–36. doi: 10.1016/j.actbio.2024.02.006
[2] ↑ Mello, T. P., Barcellos, I. C., Branquinha, M. H., and Santos, A. L. S. 2023. Cell dispersion during biofilm formation by Scedosporium apiospermum, Scedosporium aurantiacum, Scedosporium minutisporum and Lomentospora prolificans. Curr. Res. Microb. Sci. 4:100191. doi: 10.1016/j.crmicr.2023.100191
[3] ↑ Roudbary, M., Vahedi-Shahandashti, R., Santos, A. L. S. D., Roudbar Mohammadi, S., Aslani, P., Lass-Flörl, C., et al. 2022. Biofilm formation in clinically relevant filamentous fungi: a therapeutic challenge. Crit. Rev. Microbiol. 48:197–221. doi: 10.1080/1040841X.2021.1950121
[4] ↑ Mello, T., Silva, L., Ramos, L., Frota, H., Branquinha, M., and Santos, A. 2020. Drug repurposing strategy against fungal biofilms. Curr. Top. Med. Chem. 20:2–7. doi: 10.2174/156802662007200316142626
[5] ↑ Wolfmeier, H., Pletzer, D., Mansour, S. C., and Hancock, R. E. W. 2018. New perspectives in biofilm eradication. ACS Infect. Dis. 4:93–106. doi: 10.1021/acsinfecdis.7b00170
