Abstract
What a mom eats before her baby is born can impact the baby’s development. In this experiment, we fed pregnant mice an unhealthy high-fat diet to see the effect on the intestines of the baby mice after they were born. We found that the babies had more of a certain type of immune cell and that their intestines were more easily damaged compared to babies whose moms ate a healthier diet. In the second part of the experiment, we blocked the effects of these unusual immune cells and saw that the intestines of these babies became more resistant to damage, almost like those of baby mice whose moms ate healthy diets. Our research shows how important it is for moms to eat healthy foods when they are pregnant, to keep the newborn baby’s gut healthy.
Can What Mothers Eat Affect Their Babies’ Health?
Obesity is a huge problem in the United States, partly because many easily available foods have a lot of fat. Right now, more than half of the women in the United States are overweight or even obese around the age when they can get pregnant [1]. This could be dangerous, since scientists around the world have found that when moms are obese while they are pregnant, their babies have a higher risk of health problems throughout their lifetimes [1–5]. However, scientists do not yet know exactly why this happens. Even though obesity has many causes, an unhealthy, high-fat diet is one of the reasons some people are obese. This makes scientists ask: what changes happen in the baby when the mom eats a lot of fat?
Our hypothesis was that maybe, when moms eat a high fat diet, their babies will have different bacteria that may cause trouble in their intestines. This makes sense because when adults eat a lot of fat, their own gut bacteria change. Scientists believe this is because the fat encourages the growth of certain bacteria that break down the fat and allow the body to absorb more of it, making them gain more weight. With this in mind, we wondered if the bacteria of babies born to mothers on high-fat diets (60% saturated fat) can change, too [6].
Why do the bacteria in the intestines matter? These bacteria can affect the immune cells that can make the gut sensitive to damage and lead to a lot of problems. Even though the role of immune cells is to protect the body, they can also harm the body if they are too active or are activated by certain bacteria, resulting in inflammation [7]. One of the problems resulting from gut inflammation caused by specific bacteria is called necrotizing enterocolitis, which can cause babies born too soon (prematurely) to have to stay in the hospital for an extended period after they are born.
How did we test this idea? The bodies of mice have a lot of similarities with the human body, so we used mice in an experiment. Specifically, we gave some pregnant mice a diet with a lot of fat, and some mice a regular diet, with less fat. Then we looked at the guts of each of their babies to see if there were any differences. First, we looked at the babies’ gut bacteria. Second, we tested their intestinal inflammation by looking to see if they had more cells called type 3 innate lymphoid cells (ILC3 cells). These cells cause inflammation by secreting a molecule that irritates the gut and can make inflammation worse. So if we see a lot of these cells, it indicates that the mice have more gut inflammation.
What Happened to the Babies’ Gut Health?
After the pregnant mice had been on the unhealthy high-fat diet, we looked at their babies’ feces (poop) and found that the bacteria present were very different from those in the poop of the babies born from mice on a regular (healthy) diet (Figure 1).
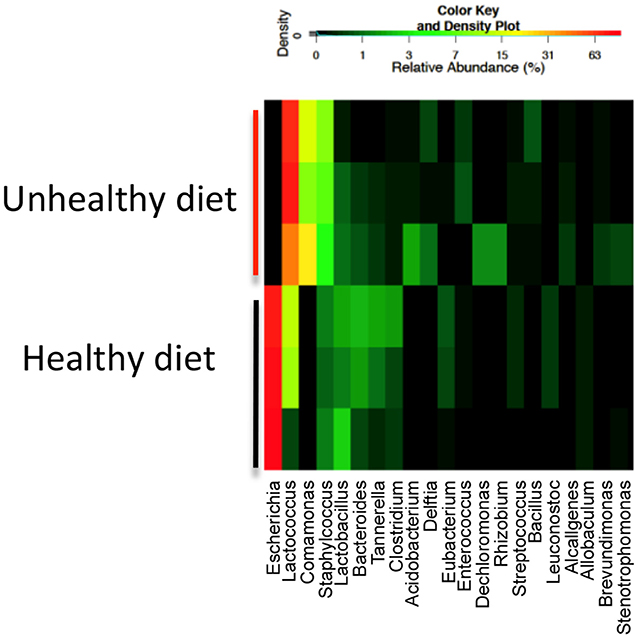
- Figure 1 - Heatmap of bacteria found in the poop of three representative 1-week-old mice whose mothers were either on a high-fat diet (unhealthy) or regular diet (healthy).
- The colors show the amounts of each bacterium (listed along the bottom), with red being more bacteria and black being less. You can see that the patterns in the unhealthy and healthy groups are quite different. This result showed us that the mom’s high-fat diet could indeed affect the intestinal bacteria of the baby mice.
We also looked at the immune cells in the babies’ intestines after they were born, and we found that ILC3 cells were increased in babies from moms that ate a high-fat diet (Figure 2). The test we used for this is called flow cytometry, in which we can identify cell types by the characteristic patterns they have on their surfaces. ILC3 cells can produce a molecule called IL-17A, which can hurt the intestine when inflammation is present.
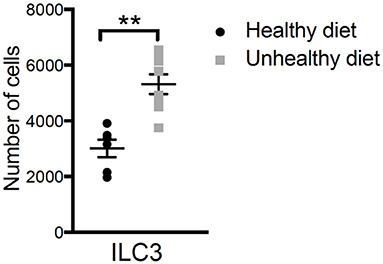
- Figure 2 - Studying the numbers of ILC3 cells in the guts of babies born to mothers on the healthy and unhealthy diets showed us that there were more ILC3 cells in the intestines of the babies born from the mothers that ate the unhealthy, high-fat diet.
- This was statistically significant as shown by the double asterisks. ILC3 cells can produce a molecule called IL-17A, which can irritate the intestine and cause inflammation. This result indicates that ILC3 may be responsible for the inflammation seen in the guts of babies born to mothers on the unhealthy diet.
Can We Protect the Babies From Intestinal Damage?
At this point, we knew that baby mice from mothers that ate an unhealthy, high-fat diet had a different pattern of gut bacteria and more dangerous ILC3 cells than babies from moms who ate a healthy diet. We wondered if we could protect the inflammation-prone babies from intestinal damage, so we did an experiment to see if we could stop the effect of the ILC3 cells—and it worked (Figure 3)! To do this, we checked the intestine of the baby mice after they had a substance that could cause inflammation to the intestine.
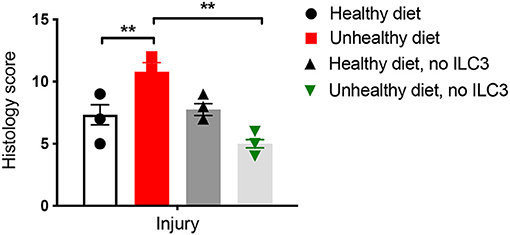
- Figure 3 - The intestines of baby mice were examined to see how much inflammation we could induce.
- This was measured by examining the intestines under a microscope (called histology) and giving the samples a score based on how much injury we saw. The higher the score, the more injury. The mice whose moms ate an unhealthy diet showed a higher score (more injury). When we blocked the effect of the ILC3 cells, the score was reduced (less injury). This told us that the ILC3 were responsible for the detrimental effects. The double asterisk represents statistical significance.
What was interesting was that, when we blocked the effect of the ILC3 cells by neutralizing IL-17A, we found we could save these babies from inflammation. This showed us that the mom’s high-fat diet changed the numbers of ILC3 cells in the babies’ intestines and caused more injury due to a higher production of IL-17A by those cells.
Mom’s Diet Matters!
This research taught us that what mothers eat while they are pregnant can affect their growing babies, even after they are born. Of course, mice are not humans, and this is a limitation of the study. However, mice are mammals, as are humans, and we can learn a lot from studying them. These experiments in mice showed us that the high-fat diet during pregnancy changed the immune cells and bacteria in the guts of the baby mice. Our findings could mean that eating healthy and avoiding high-fat foods when pregnant is very important for humans, too. A healthy diet does include some fat, but not an excessive amount. Eating healthy is all about getting a balance of all the nutrients that we need in our bodies, including vitamins, carbohydrates, proteins, healthy fats, and minerals.
Glossary
Intestine: ↑ A part of the gut where food is broken down and absorbed. Many bacteria live in the intestine.
Saturated Fat: ↑ A type of fat, that can be eaten, that has a high proportion of fatty acid molecules and is considered unhealthy.
Immune Cells: ↑ Cells that help protect the body from dangerous invaders such as harmful bacteria and viruses.
Inflammation: ↑ The state that results when immune cells are activated and cause damage to other cells. Inflammation appears as redness, swelling, heat, and pain.
Necrotizing Enterocolitis: ↑ A disease in which the intestines of babies that are born too soon (premature) are damaged, causing the babies to stay in the hospital for a while.
Type 3 Innate Lymphoid Cells: ↑ A type of immune cell present in the intestine that can produce the cytokine IL-17A.
Flow Cytometry: ↑ A technology that uses lasers to analyze single cells based on the molecules on their surfaces.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This research was originally funded by NIH K08 (DK100545), a Young Investigator Grant for Probiotic Research and a Children’s Medical Center Foundation Grant to JM. We would also like to acknowledge Sameera Chiruvolu (Ursuline Academy of Dallas) for her help in editing the manuscript.
Original Source Article
↑Babu, S. T., Niu, X., Raetz, M., Savani, R. C., Hooper, L. V., and Mirpuri, J. 2018. Maternal high-fat diet results in microbiota-dependent expansion of ILC3s in mice offspring. JCI Insight. 3:e99223. doi: 10.1172/jci.insight.99223
References
[1] ↑ Deputy, N. P., Dub, B., and Sharma, A. J. 2018. Prevalence and trends in prepregnancy normal weight-48 states, New York City, and District of Columbia, 2011–2015. Morb. Mortal Wkly Rep. 66:1402–7. doi: 10.15585/mmwr.mm665152a3
[2] ↑ Journault, M., Murthy, P., Bansal, N., Tang, S., Al Awad, E., Creighton, D., et al. 2023. The association of maternal overweight on long-term neurodevelopmental outcomes in premature infants (<29 weeks) at 18–24 months corrected age. J. Perinatol. 43:1413–9. doi: 10.1038/s41372-023-01733-1
[3] ↑ Denizli, M., Capitano, M. L., and Kua, K. L. 2022. Maternal obesity and the impact of associated early-life inflammation on long-term health of offspring. Front. Cell Infect. Microbiol. 12:940937. doi: 10.3389/fcimb.2022.940937
[4] ↑ Sridhar, S. B., Darbinian, J., Ehrlich, S. F., Markman, M. A., Gunderson, E. P., Ferrara, A., et al. 2014. Maternal gestational weight gain and offspring risk for childhood overweight or obesity. Am. J. Obstet. Gynecol. 211:259.e1–8. doi: 10.1016/j.ajog.2014.02.030
[5] ↑ Catalano, P. M., and Ehrenberg, H. M. 2006. The short- and long-term implications of maternal obesity on the mother and her offspring. BJOG 113:1126–33. doi: 10.1111/j.1471-0528.2006.00989.x
[6] ↑ Aron-Wisnewsky, J., Warmbrunn, M. V., Nieuwdorp, M., and Clement, K. 2021. Metabolism and metabolic disorders and the microbiome: the intestinal microbiota associated with obesity, lipid metabolism, and metabolic health-pathophysiology and therapeutic strategies. Gastroenterology 160:573–99. doi: 10.1053/j.gastro.2020.10.057
[7] ↑ Belkaid, Y., and Harrison, O. J. 2017. Homeostatic immunity and the microbiota. Immunity 46:562–76. doi: 10.1016/j.immuni.2017.04.008
