Abstract
Water pollution is a very concerning problem since it affects millions of people around the world. Arsenic is a highly toxic metal that can cause serious health problems if consumed. Substances called metal oxides can help to remove contaminants like arsenic from water, and researchers have recently shown that these superheroes are even more powerful when they are combined. In anime, the superheroes Goku and Vegeta can fuse to become Gogeta, and when they do so their power increases. Similarly, combining metal oxides is a superpower that could save the world from poisonous arsenic, through a trapping mechanism called adsorption. In this article, we will explain what arsenic is, how it can affect humans, and how metal oxides might eventually help us to produce safer water.
Water: Important for All Living Things
Water is one of the most important natural resources. It is critical for the survival of living beings and for the development of societies. However, water is not equally distributed across the world—some regions are extremely humid and others are very dry. In addition, the demand for water has increased as the human population has grown. To meet the growing need, technology has been used to extract water from much deeper aquifers. However, this water often contains high concentrations of substances such as arsenic from the rocks surrounding the aquifers, which make it undrinkable. At the same time, human activities have also contributed to contamination of water with elements such as arsenic, mercury, cadmium, chromium, and lead. Among these elements, arsenic appears to be the most toxic to humans and other animals [1].
What Is Arsenic and How Does It Affect Humans?
Arsenic is a natural element of the Earth’s crust, and it is found in the air, water, and soil. Arsenic can exist in two forms: organic and inorganic. Inorganic arsenic compounds found in water are highly toxic, while the organic compounds are less toxic and are found in fish and shellfish. Exposure to high levels of inorganic arsenic usually happens through drinking contaminated water or the use of contaminated water for food preparation and irrigation of food crops. Human exposure to arsenic has become a major global concern.
Long-term ingestion of arsenic-contaminated water, even in very low amounts, can pose health risks (Figure 1) [2]. According to the World Health Organization (WHO), exposure to low amounts of arsenic over ~5 years can cause skin pigmentation changes, skin lesions, and thick calluses on the palms of the hands and soles of the feet. In addition, arsenic can cause skin, bladder, and lung cancer. The WHO recommends limiting inorganic arsenic to 10 micrograms per liter (μg/L) of water for human consumption. Natural contamination of groundwater with arsenic has been reported worldwide, with concentrations well above the maximum permissible limit. Arsenic concentrations of up to 25 μg/L have been reported in groundwater in parts of Mexico [3]. In other countries, such as Bolivia, Bangladesh, Cambodia, Taiwan, Pakistan, Thailand, and Vietnam, arsenic concentrations of 45.9, 4,600, 178, 1,800, 2,580, 5,000 and 159 μg/L, respectively, have been found in groundwater [3].
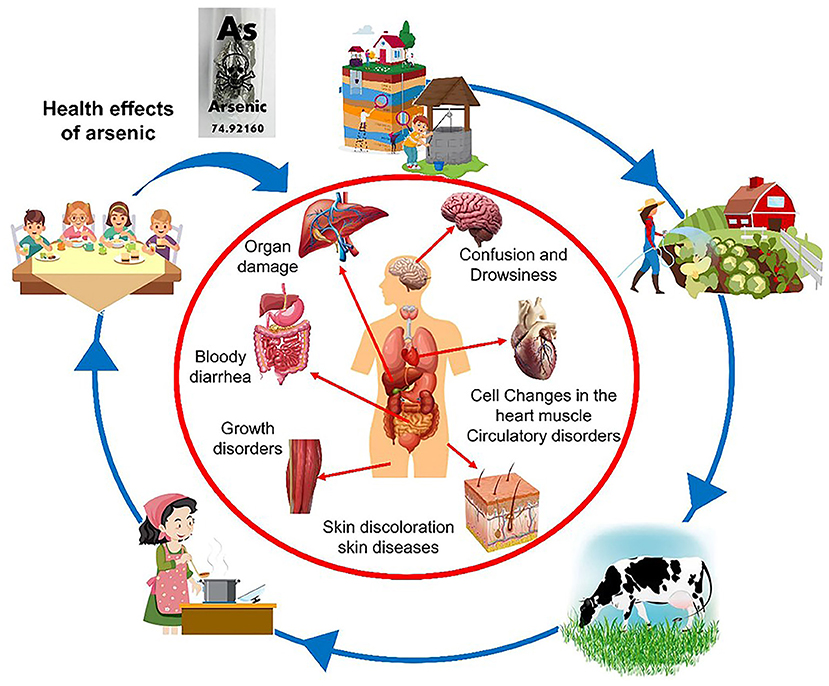
- Figure 1 - The main way that arsenic gets into humans is through water contaminated with arsenic.
- The use of arsenic-contaminated water to irrigate crops, prepare food, and as drinking water for animals and humans can cause serious health problems and even cancer.
Metal Oxides: Heroes to the Rescue
Metal oxides are compounds that result from the combination of a metal with a non-metal, such as oxygen. The reddish, powdery rust you may have seen on a metal object left outdoors is an example of a metal oxide. When metal oxides are placed in water, their surfaces can stick to contaminants that have a negative charge, like arsenic. This quality is interesting to researchers because it means that metal oxides may be able to purify water by removing contaminants. For example, metallic oxides of cerium (CeOx) and iron (FeOx) have been shown to be very good at removing arsenic from water.
If you are familiar with Dragon Ball anime, you could imagine that Goku is CeOx and Vegeta is FeOx. Both oxides have the power to capture various contaminants from water, including arsenic, the same way that Goku and Vegeta have the power to defeat the evildoers who threaten the wellbeing of the Earth. You may remember that Goku learns a technique known as the Fusion Dance, which can unite two bodies into a single being. Similarly, in recent years there has been a special interest in combining metallic oxides for the removal of arsenic from water. This is because these bimetallic oxides are even better at removing contaminants from water than either oxide is on its own [4]. In other words, the metallic oxides CeOx and FeOx increase their arsenic-capture power by merging to create CeOx:FeOx, which is similar to Goku and Vegeta who, by merging to become Gogeta, increase their powers to defeat the evil ones who threaten Earth. To achieve this, Goku and Vegeta must perfectly align their fingers, and the positions of their legs and arms must have the same angle (Figure 2A).
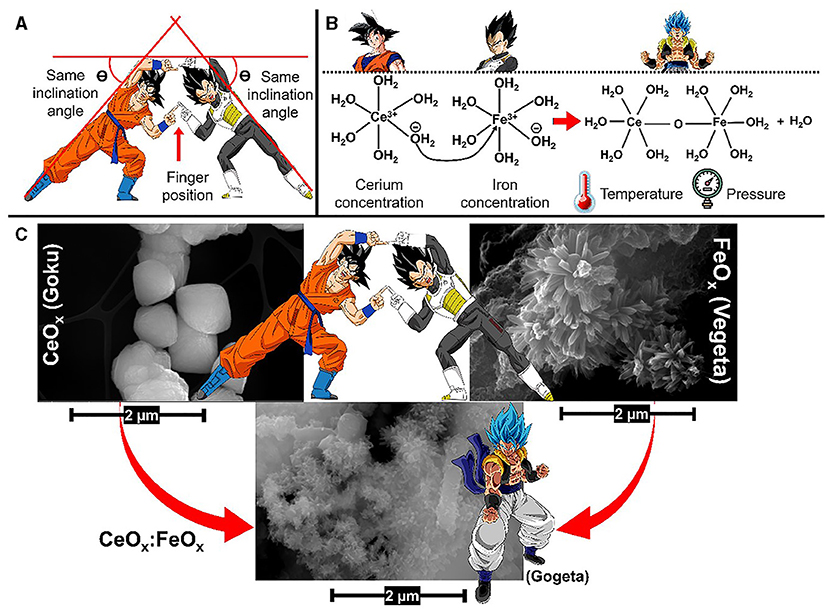
- Figure 2 - (A) Goku and Vegeta fuse to become Gogeta by aligning their fingers and the angels of their arms and legs.
- (B) In a similar way, the bimetallic oxide CeOx:FeOx is formed when the optimal temperature, pressure, and concentrations of cerium and iron oxide are present. (C) SEM images of CeOx, FeOx, and the bimetallic oxide CeOx:FeOx (Dragon Ball images are property of Akira Toriyama).
To create bimetallic oxides, it is important for researchers to determine the optimal parameters, such as temperature, pressure, and concentration of each metal oxide (Figure 2B).
Figure 2C shows the structure of these materials seen using scanning electron microscopy (SEM). Cerium oxides (CeOx) have an octahedral (eight-sided) structure and iron oxides (FeOx) appear as flower-shaped structures. However, the bimetallic oxide (CeOx:FeOx) has a completely different structure that looks like a bunch of tiny needles sticking together in cloud-like shapes [5]. The SEM micrograph of CeOx:FeOx (Gogeta) shows many tiny branches, providing lots of surface area for arsenic to stick to.
How Do Bimetallic Oxides Remove Arsenic From Water?
Broly is a very powerful villain and, even when he was just a baby, his immense power caused fear in those around him. Similar to Broly, arsenic is dangerous because it can cause severe damage to humans even at very low concentrations. Therefore, Goku (CeOx) and Vegeta (FeOx) join forces to defeat the powerful villain Broly (arsenic). Bimetallic oxides remove arsenic through a process called adsorption. Adsorption is process in which one or more substances present in a fluid (liquid or gas) accumulate on a surface and are thus removed from the fluid. Basically, pieces of the bimetallic oxide molecules are replaced by arsenic, through a reaction called ligand exchange (Figure 3). Once the arsenic binds to the bimetallic oxide, it is removed from the water and water molecules are formed by the reaction [5]. This produces arsenic-free water that complies with the maximum limit of 10 μg/L recommended by the WHO.
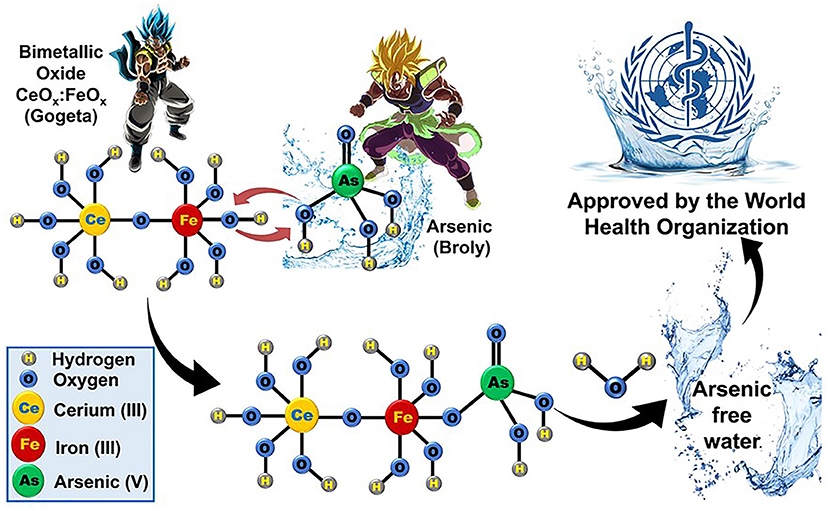
- Figure 3 - Just as Gogeta can defeat the evil villain Broly, the bimetallic oxide CeOx:FeOx can bind to arsenic and remove it from water.
- Water is also produced in that reaction. The levels of arsenic in the purified water are within the limits recommended by the World Health Organization (Dragon Ball images are property of Akira Toriyama).
How Will Bimetallic Oxides Be Used in Water Purification?
Scientific advances in the synthesis of adsorbent materials, such as CeOx:FeOx not only solve an environmental problem (arsenic contamination of aquifers) but also prevent the poisoning of animals and humans. Bimetallic oxides are highly promising for removing various contaminants from water, but doing so on a large scale remains very challenging. Currently, the tiny size of these metal oxides makes it difficult to use them in typical water filtration systems. Scientists are working on attaching bimetallic oxides to a matrix, kind of like a scaffold, which might allow them to be used in water purification [6]. Much more work is needed to continue to develop new, efficient materials that can combat water pollution and protect the health of the environment and humanity.
Glossary
Aquifers: ↑ Water reservoirs located below the Earth’s surface, where water circulates through cracks in rocks.
Metal Oxide: ↑ Combination of a metal (such as Ce or Fe) with oxygen.
Bimetallic Oxide: ↑ Combination of two different metals (such as Ce and Fe) with oxygen.
Scanning Electron Microscopy: ↑ A widely used technique for examining and analyzing extremely tiny objects that cannot be seen with a “regular” light microscope.
Surface Area: ↑ The total area of a solid material, including the area within any pores.
Adsorption: ↑ When molecules, ions, or compounds in a solution stick to the surface of a solid material.
Ligand Exchange: ↑ When a molecule exchanges part of itself with another molecule, to form a new compound or molecule.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was financially supported by the CONAHCYT project SEPCB-2014-01-237118 and FORDECYT-2018-8-297525.
References
[1] ↑ Shaji, E., Santosh, M., Sarath, K. V., Prakash, P., Deepchand, V., and Divya, B. V. 2021. Arsenic contamination of groundwater: a global synopsis with focus on the Indian Peninsula. Geosci. Front. 12:101079. doi: 10.1016/j.gsf.2020.08.015
[2] ↑ Patel, R., Shah, D., Shah, S., and Shah, M. 2022. Green nanomaterials for removal of arsenic and fluoride contamination from wastewater. Mater. Today Proc. 62:7318–23. doi: 10.1016/j.matpr.2022.05.100
[3] ↑ Shaji, E., Santosh, M., Sarath, K. V., Prakash, P., Deepchand, V., and Divya, B. V. 2021. Arsenic contamination of groundwater: a global synopsis with focus on the Indian Peninsula. Geosci. Front. 12:101079. doi: 10.1016/j.gsf.2020.08.015
[4] ↑ Hristovski, K., Baumgardner, A., and Westerhoff, P. 2007. Selecting metal oxide nanomaterials for arsenic removal in fixed bed columns: from nanopowders to aggregated nanoparticle media. J. Hazard. Mater. 147:265–74. doi: 10.1016/j.jhazmat.2007.01.017
[5] ↑ Vences-Alvarez, E., Chazaro-Ruiz, L. F., and Rangel-Mendez, J. R. 2022. New bimetallic adsorbent material based on cerium-iron nanoparticles highly selective and affine for arsenic(V). Chemosphere 297:134177. doi: 10.1016/j.chemosphere.2022.134177
[6] ↑ Rios-Saldaña, L. E., Pérez-Rodríguez, F., Vence-Alvarez, E., Nieto-Delgado, C., and Rangel-Mendez, J. R. 2022. Synthesis of a granular composite based on polyvinyl alcohol-Fe:Ce bimetallic oxide particles for the selective adsorption of As(V) from water. J. Water Process. Eng. 46:102621. doi: 10.1016/j.jwpe.2022.102621
