Abstract
Carbon dioxide (CO2) plays an important role on Earth. But right now, there is too much! Burning fossil fuels increases the CO2 emissions into the atmosphere, causing global warming. Friend or foe, reducing CO2 is crucial. One way to do this is to capture CO2 before it is released from factory chimneys, or to take it directly from the air. These strategies not only reduce CO2, but also offer a way to reuse it in daily life products. Can you imagine captured CO2 being used to create the bubbles in your soda or growing the vegetables you eat? Current efforts are focused on designing new materials that can powerfully attract CO2. These materials have spaces inside where CO2 can enter and stick to the surface through a process called adsorption. The objective is to capture CO2 that can later be released to use for other purposes!
Friend or Foe?
Carbon dioxide (CO2) has been in the atmosphere since the Earth was a young planet. At that time, there were a lot of volcanoes that released water steam, nitrogen, and CO2 in huge amounts. A lot of the CO2 was dissolved into the oceans until microbes used sunlight to convert it into oxygen and sugars through photosynthesis. This oxygen accumulated in the atmosphere, bubble by bubble, until many types of organisms such as plants and large animals evolved. Surprisingly, humans exist because of CO2 and its conversion to oxygen!
Furthermore, CO2 plays an important role in our climate because it is one of the greenhouse gases (GHGs) that trap the heat from the sun. The greenhouse effect is a natural process that keeps Earth’s climate comfy for living things. Without it, Earth would be very cold, and many life forms would freeze. For 20 million years, the amount of CO2 was below 280 parts per million (ppm). However, human activities have increased the amounts of CO2 and other GHGs in the atmosphere. Too many GHGs cause the atmosphere to trap more heat, warming the Earth. This rise in global temperature is called global warming. Burning fossil fuels like coal, oil, and natural gas have increased CO2 emissions by 50% in <200 years, reaching levels of 417 ppm [1]—the highest CO2 levels in human history! At the same time, the average global temperature has increased 1.26°C over the past 150 years. 2020 was the warmest year on record! You can probably see the effects of global warming where you live: hot days, extreme rainfalls, melting ice, and changes in flower blooming times. What will happen if CO2 emissions in the atmosphere continue to increase?
CO2 Emissions: Filling Up the “Bathtub”
The presence of CO2 into our atmosphere is like running water in a bathtub with the drain open (Figure 1). There is a tap continuously adding CO2 to the tub, from burning fossil fuels and forests, plant and animal respiration, volcano eruptions, and wildfires. But there is also a natural drain that absorbs CO2 through plants, the ocean, and the land. Currently, burning fossil fuels and emissions from industrial factories are adding more CO2 than the natural drain can handle, clogging the drain a bit and filling the tub. What would happen if the tub filled up? Imagine yourself in the bathtub enjoying a bubble bath. To begin filling the tub, you should probably close the drain and turn on the tap. But what if you forget to turn off the tap? Your bathroom will certainly be flooded, as soapy water overflows the tub! According to scientists, if we do not reduce CO2 emissions and emissions continue to increase after 2050, the global average temperature will increase between 4.5 and 5°C [2]. This may not sound like much, but even this increase could cause heat waves, heavy rainfalls, species extinctions and habitat loss, ocean acidity, coral reef loss, and human health risks. For that reason, we need to reduce CO2 emissions and create new “artificial drains” that can prevent the overflow.
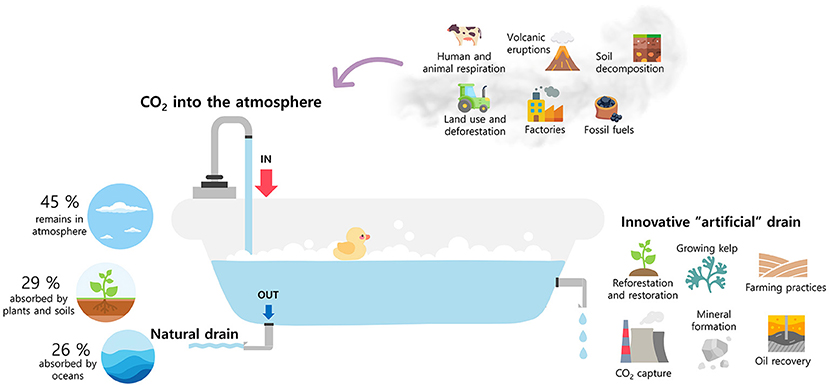
- Figure 1 - You can think of CO2 in the atmosphere like a bathtub with an open drain.
- Human activities are putting more CO2 into the tub than the natural drain can handle, filling the tub. If atmospheric CO2 concentrations continue to increase, the bathtub will overflow. This means that the global average temperature will increase too, and the world will experience many negative consequences of climate change. Creating new “artificial drains” can reduce CO2 emissions and prevent overflow, helping to prevent global warming (Figure concept adapted from Sterman’s Carbon Bathtub).
How to Remove and Store CO2?
There are many ways to create “artificial drains” to remove CO2 from the atmosphere. Reforestation, restoration of mangroves and seagrass, growing kelp, certain types of farming practices, and several advanced technologies are some ideas. While some technologies are at an early stage, they can become possible with specialized research. Particularly, carbon capture, utilization, and storage (CCUS) systems involve technologies for catching the CO2 emitted by power plants (where electricity is produced) and large industrial factories, so that it is not released into the atmosphere [3]. Scientists have developed a few ways to catch CO2. The most common is capturing it directly from flue gases that result from burning fossil fuels (Figure 2).
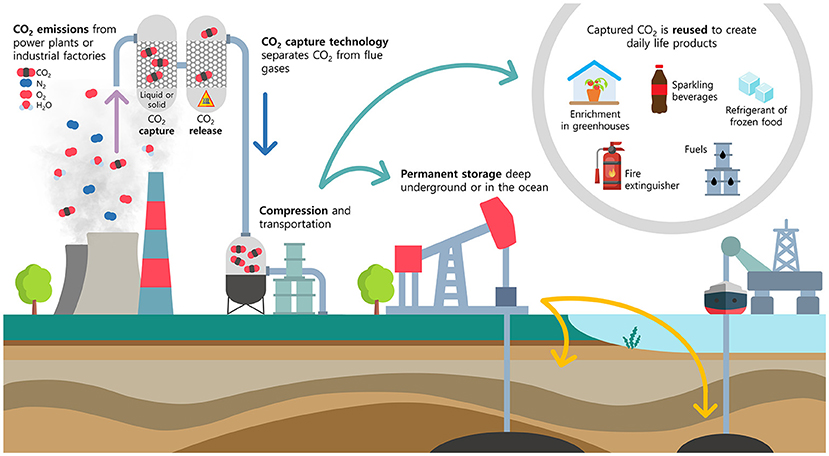
- Figure 2 - Carbon capture, utilization, and storage (CCUS) systems could serve as an “artificial drain” to reduce CO2 levels in the atmosphere.
- Flue gases from a chimney, which typically contain 3%-15% CO2, are sent to a tower where the CO2 is captured by a liquid or solid material. Once CO2 is captured, it must later be released and compressed for permanent storage or for reuse in daily life products.
Flue gases are a mixture of gases that flow through a chimney as a result of burning, and they typically contain 3%-15% CO2 along with nitrogen, water steam, oxygen, particles, and other substances. Have you ever seen a powerful factory chimney spewing out smoke? If a factory uses a CCUS system, replacing factory chimneys with capture towers, its CO2 emissions might be reduced by 80%-90%! In a CCUS system, flue gases from a chimney are sent to a tower, where CO2 is captured by a liquid or solid material. Another tower takes the CO2 out of the material so that it can be used again. Once CO2 is captured, it must be compressed to transport it by a pipeline to a place where it can be stored safely, perhaps permanently. Storing CO2 underground, deep in the ocean, or turning it into minerals such as cement are some ideas for safe storage that are being studied.
Furthermore, after CO2 is captured, it can be used to create products that we use in our daily lives. Do you know that tons of CO2 are used to help grow vegetables? When CO2 is pumped into a greenhouse, the tomatoes, cucumbers, and lettuce crops produced can grow bigger, better, and tastier. CO2 can also be used to produce sparkling beverages, as a refrigerant for frozen food, and as a fire extinguisher. CO2 can even be converted to methanol, a type of alcohol used to create fuels for vehicles and boats, or to make adhesives.
CO2 Capture With More Carbon!
The first and largest CO2 capture and storage system in the world is in Iceland. It is called Orca. Currently, it removes up to 4,000 tons of CO2 annually, directly from the air—the same amount that 145,000 trees can absorb! CCUS usually catch CO2 using liquid chemicals called amines. But it requires a large amount of energy to release CO2 from amines so that both can be used again. The overall efficiency of a power plant could be reduced by 7%-12% because of the heat and power required for the CCUS! For that reason, our research group and other scientists are designing new CO2-capturing materials that can be reused many times and need less energy. Some of these materials are made of carbon atoms. What a coincidence! Solid forms of carbon are usually black materials. Each member of the carbon family is unique in terms of shape, texture, and ability to catch foreign atoms. The diamond in a ring, the graphite in a pencil, the charcoal in a barbecue, and the activated carbon in an aquarium are some members of this family. These substances are made of carbon atoms connected in a hexagonal shape (Figure 3A), and a single layer of carbon atoms connected this way is called graphene [4].
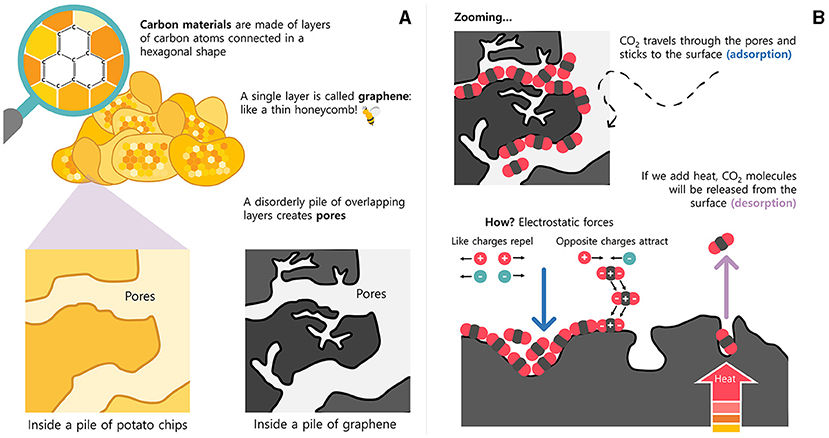
- Figure 3 - (A) Some carbon materials are black solids made of disordered layers of carbon atoms, like a pile of potato chips.
- The spaces in between the layers are called pores. (B) To remove CO2 from flue gases, the gases travel through the carbon material and, in the pores, the CO2 becomes stuck to the surface by electrical charges called electrostatic forces. Later, heat can be applied to release CO2 from the surface, so it can be concentrated and stored or reused.
Observed under a powerful microscope, graphene resembles an extremely thin honeycomb: a million times thinner than a human hair! If the graphene layers overlap with one another in a disorderly way (like a pile of potato chips), spaces are created, which are called pores. To remove CO2 from flue gases, the pore size must be similar to the size of the CO2 molecule. Once inside, CO2 travels through the pores and sticks to the surface in a process called adsorption (Figure 3B). The consequence is a very thin layer of molecules stuck on the surface, due to weak electrostatic forces. These forces are caused by attractive and repulsive interactions due to particles’ natural electric charges. To remove CO2 from the surface, we can supply heat or a vacuum to break the electrostatic interactions. This process is called desorption. The CO2 can then be concentrated, and the graphene layers can be reused.
Take-Home Message
Currently, our research group works on the design of carbon materials with a high capacity to capture CO2. The higher the attraction for CO2, the more gas can be captured in a very short time. Likewise, the combination of carbon surfaces with liquids or small metallic particles could increase the CO2 capture by combining their gas-attraction superpowers. It may also be possible to “grow” other carbon materials over the graphene surface to increase the spaces where CO2 can be caught. These studies play an important role in steps to combat global warming and its negative impacts. However, it is important to remember that CCUS systems alone will not solve the problem. Transportation, manufacturing, and construction are significant contributors of CO2 to the atmosphere and substantial consumers of energy. So, creating buildings with longer lifespans, switching to hybrid or electric cars, and using recycled materials are some other important steps that must be taken to reduce CO2 levels. You can also make a difference from home! Just practice turning the lights off, taking short showers, riding a bike instead of taking a car when possible, throwing away less food, being more conscious about your eating habits, recycling… and most importantly, keep learning!
Glossary
Carbon Dioxide: ↑ Molecule made by one carbon atom and two oxygen atoms. It is a greenhouse gas that traps heat close to Earth.
Carbon Capture, Utilization, and Storage (CCUS): ↑ Technologies that capture CO2 directly from the air or from power plants and factories before it is released into the atmosphere.
Amines: ↑ Molecules that contain a nitrogen atom, capable of forming new bond with other molecules. They are applied for CO2 capture.
Graphene: ↑ An atomic-scale honeycomb net made of carbon atoms. It is one of the forms of carbon.
Pores: ↑ Minuscule openings or cavities within solid materials that allow gases or liquids to pass easily.
Adsorption: ↑ Sticking of atoms or molecules to a solid surface.
Electrostatic Forces: ↑ Attractive or repulsive forces between particles that are caused by their natural electrical charges.
Desorption: ↑ Releasing of atoms or molecules from a solid surface through methods such as vacuum, heating, or chemical reactions.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was financially supported by the CONAHCYT scholarship CVU 901672.
References
[1] ↑ WMO - World Meteorological Organization. 2022. Greenhouse Gas Bulletin No. 18. Geneva: World Meteorological Organization, p. 1–10.
[2] ↑ Intergovernmental Panel on Climate Change. 2019. Global warming of 1.5°C. Special Report on Global Warming of 1.5°C. Geneva: Intergovernmental Panel on Climate Change.
[3] ↑ Intergovernmental Panel on Climate Change. 2005. Carbon Dioxide Capture and Storage. Vol. 33. Geneva: Intergovernment Panel on Climate Change.
[4] ↑ Menéndez-Díaz, J. A., and Martín-Gullón, I. 2006. “Types of carbon adsorbents and their production”, in Actived Carbon Surfaces in Environmental Remediation, Vol. 7, Interface Science and Technology, ed. T. J. Bandosz (Edinburgh: Elsevier Science), p. 1–47.
