Abstract
Legionella pneumophila is type of bacteria present in natural water sources, such as rivers, ponds, and in man-made water reservoirs, such as water fountains. Amoebas are single-celled organisms that also live in water sources and generally feed on bacteria. However, Legionella are not digested by amoebas and have even evolved to multiply inside the amoebas that try to digest them! This resistance to being eaten by amoebas has enabled the Legionella bacteria to survive in water sources and to be transmitted to humans through contaminated water droplets. Their ability to survive inside amoebas also allows Legionella bacteria to survive and multiply within cells in the human lungs, leading to pneumonia. Therefore, the long relationship between Legionella and amoebas helped Legionella to evolved and to infect human macrophages causing disease.
What is Legionnaires’ Disease?
In the summer of 1976, an unknown disease began spreading among hotel guests in the American city of Philadelphia [1–3]. Symptoms included feeling short of breath, coughing, chills, headache, chest pain, and sometimes diarrhea. One hundred eighty people were infected and 29 of them died as a result of this mysterious disease, including several members of the American Legion, an organization for US war veterans.
The US Centers for Disease Control and Prevention (CDC) immediately stepped in to determine the cause of this mysterious disease. Researchers took samples of lung tissue from the deceased victims and tried to figure out whether the infectious agent was an inhaled bacteria, virus, or fungi. After 5 months of experiments, the scientists identified the cause of the mysterious epidemic, and to their surprise it was a bacteria normally present in nature that was not previously known to infect humans. The scientists called it Legionella pneumophila—“Legionella” for the American Legionnaires who were infected, and “pneumophila” to indicate its infection of the lungs. The disease caused by these bacteria was termed Legionnaires’ disease.
But how were the patients infected with these bacteria? Researchers knew that, in nature, Legionella is found in watery environments like rives and swamps. So, one theory was that patients inhaled water mist contaminated with pneumonia-causing Legionella, either inside the hotel or in the surrounding area. After collecting samples from water tanks, humidifiers, and water-cooling towers in the hotel’s air-conditioning system, it was found that the cooling towers of the hotel’s central air-conditioning system were contaminated with this Legionella pneumophilia.
From Amoebas to Humans
But why did humans suddenly become a new target for Legionella? To answer this question, we must go back millions of years, when Legionella existed in its natural aquatic environment with many other microorganisms, including a single-celled organism called an amoeba. Amoebas primarily eat bacteria present in their aquatic environments. Amoebas eat bacteria by surrounding them and bringing them inside the amoeba, creating a membrane-enclosed compartment called a food vacuole, the bacterium then is broken down by digestive enzymes inside the vacuole (Figure 1A). However, over millions of years, Legionella bacteria evolved in ways that helped them to avoid being eaten by amoebas—and even to control and manipulate many cellular processes within the amoeba! For example, Legionella bacteria evolved the ability to survive and reproduce inside of amoebas (Figure 1B), and inside other amoeba-like organisms including cells called macrophages, which are part of the body’s immune system and can be found in human lungs (Figure 1C) [4].
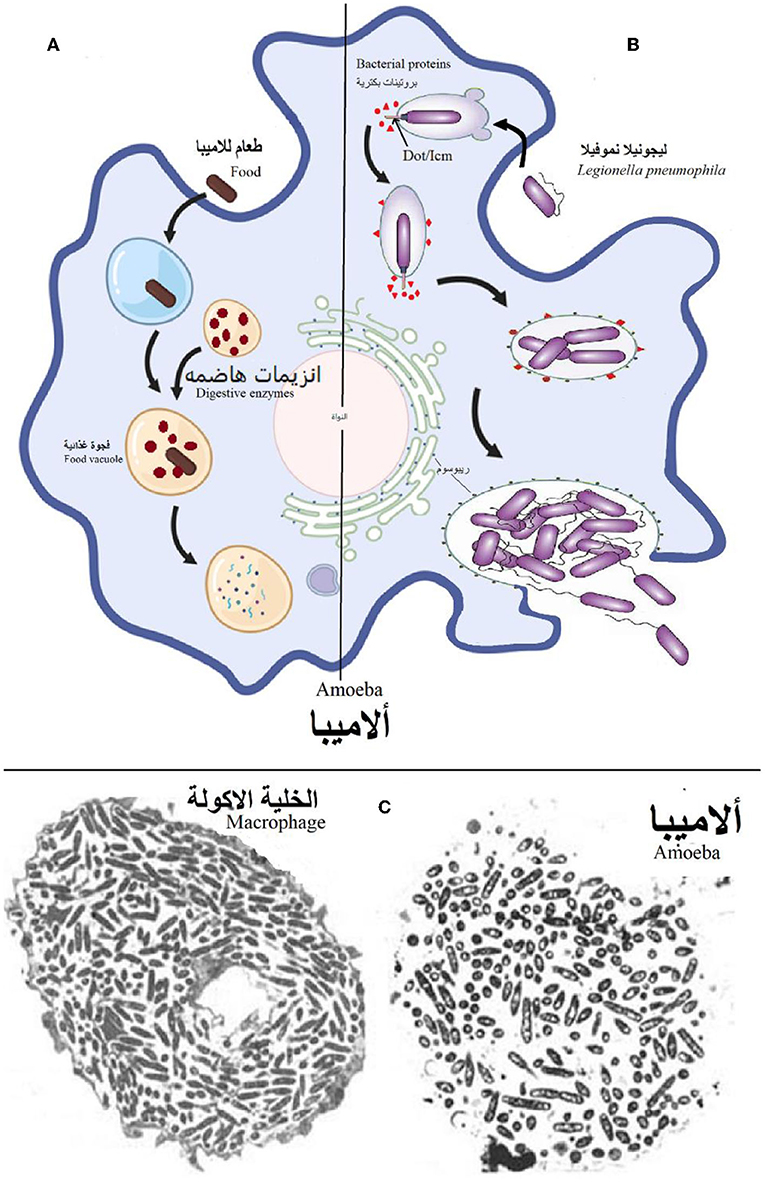
- Figure 1 - (A) When an amoeba ingests food (including most bacteria) from the surrounding environment, the amoeba breaks down the food using digestive enzymes, inside a food vacuole.
- (B) However, when an amoeba ingests Legionella pneumophila, the bacteria rapidly inject a large group of bacterial proteins into the amoeba, via the Dot/Icm “injector.” These proteins target cellular processes within the amoeba and prevent the Legionella from being digested—instead, creating an environment that is suitable for their multiplication. After multiplying in large numbers, the Legionella bacteria emerge from the amoeba, ready to infect new cells and repeat the process. (C) Electron microscope images showing the multiplication of Legionella within a macrophage (left) and amoeba (right).
The development of artificial water systems, such as air conditioning systems, cooling towers, fountains, showers, and other devices that release spray, have helped Legionella to infect humans. When a person inhales water spray contaminated with Legionella pneumophila, the bacteria reach the lungs, and as a defensive mechanism, the immune cells present in the lungs—specifically macrophages—attack and devour the Legionella pneumophila to eliminate it. Since Legionella pneumophila has evolved to survive in amoeba-like cells, it begins to control the macrophages and multiply within them. This leads to pneumonia.
Elderly people and people with weakened immune systems are at the highest risk of developing Legionnaires’ disease [5]. Fortunately, infected people can recover by taking antibiotics—medicines that kill bacteria. Legionella bacteria cannot spread between humans, so infected people cannot transmit the disease to healthy people.
How Does Legionella Take Control of Cells?
How does Legionella pneumophila manage to survive and multiply inside amoebas and macrophages? Scientists have discovered that one of the most important factors is an integrated structure in the plasma membrane of Legionella, called Dot/Icm (Figure 2). This structure looks like a needle, and it is used to inject more than 350 bacterial proteins from the Legionella into the amoeba or macrophage. These bacterial proteins interfere with the normal cellular activities of amoebas or macrophages, preventing those cells from digesting Legionella and creating the conditions that allow the Legionella to survive and multiply. To understand how those bacterial protein can control cellular activities of amoebas or macrophages, studying the DNA of Legionella pneumophila was essential. Therefore, scientists discovered that 5% of Legionella pneumophila DNA codes for bacterial proteins are similar in structure and function to proteins found in the amoebas or macrophages they infect. These similar proteins probably play a role in interfering with the cellular functions of infected cells, allowing Legionella to survive and replicate.
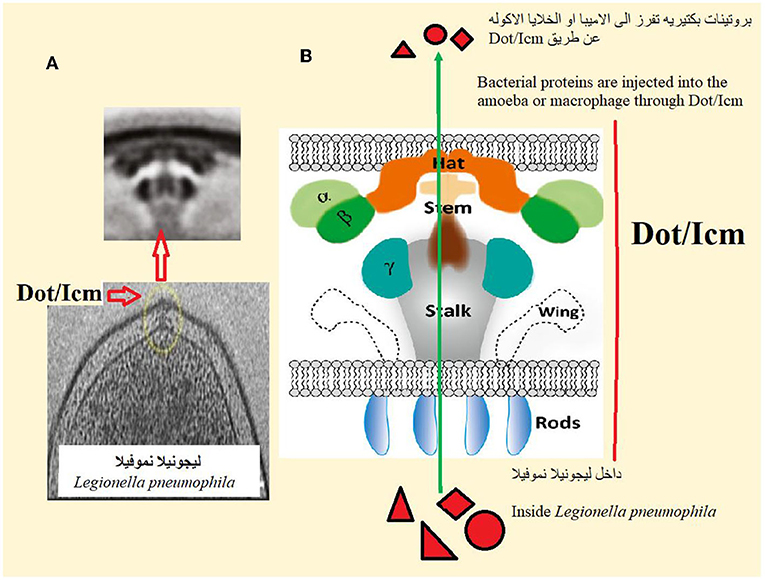
- Figure 2 - (A) Dot/Icm is a structure on the surface of Legionella pneumophila that “injects” bacterial proteins directly into amoeba or macrophage.
- The upper image is an enlargement of the circled area in the lower image. (B) Dot/Icm consists of several parts that work together to allow it to inject bacterial proteins.
Where did these important genes—so similar to those of amoebas and macrophages—come from? Over millions of years, Legionella likely acquired those genes after it was taken up by amoebas, or from the watery environment in which both organisms were living. Legionella is known to be able to uptake pieces of DNA that are present in the watery environments, regardless of the type of organism they come from!
Conclusion
Legionella pneumophilia is a fascinating organism that exists naturally in watery environments. But, with the development of artificial water systems over the past few decades, the chances of human exposure to water spray contaminated with Legionella has increased. Since the macrophages in human lungs are similar to the amoebas that Legionella has evolved to infect in nature, some of the proteins produced by these bacteria can control the cellular activities of macrophages, allowing Legionella to avoid digestion and multiply within human macrophages, leading to pneumonia. It can be said that the long relationship between amoebas and Legionella helped Legionella to both survive in nature and to infect (and cause disease in) humans.
Glossary
Amoeba: ↑ An irregularly shaped, single-celled organism often living in soil, and warm fresh water.
Food Vacuole: ↑ Food vacuole is a membrane-enclosed sac, formed by the outer membrane of the cell after phagocytosis. The vacuole contains digestive enzymes, which break down the food and then it is released into the cytoplasm for utilization. Food vacuole is present in unicellular protozoans such as amoeba.
Macrophages: ↑ Cells of the immune system, whose main function is to swallow and digest foreign bodies such as bacteria and viruses.
Immune System: ↑ Immune system is a complex network of cells, tissues, and organs. Together they help the body fight off the germs (bacteria or viruses) that invade your body.
Antibiotics: ↑ Antibiotics are medicines that fight bacterial infections in people and animals. They work by killing the bacteria or by making it hard for the bacteria to grow and multiply.
Dot/Icm: ↑ Defect in organelle trafficking/intracellular multiplication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
[1] ↑ Al-Quadan, T., Price, C., and Abu Kwaik, Y. 2012. Exploitation of evolutionarily conserved amoeba and mammalian processes by Legionella. Trends Microbiol. 20:299–306. doi: 10.1016/j.tim.2012.03.005
[2] ↑ Abu Kwaik, Y., Gao, L. Y., Stone, B. J., Venkataraman, C., and Harb, O. S. 1998. Invasion of protozoa by Legionella pneumophila and its role in bacterial ecology and pathogenesis. Appl. Environ. Microbiol. 64:3127–33. doi: 10.1128/AEM.64.9.3127-3133.1998
[3] ↑ Fraser, D. W., Tsai, T. R., Orenstein, W., Parkin, W. E., Beecham, H. J., Sharrar, R. G., et al. 1977. Legionnaires’ disease: description of an epidemic of pneumonia. N. Engl. J. Med. 297:1189–97. doi: 10.1056/NEJM197712012972201
[4] ↑ Molmeret, M., Horn, M., Wagner, M., Santic, M., and Abu Kwaik, Y. 2005. Amoebae as training grounds for intracellular bacterial pathogens. Appl. Environ. Microbiol. 71:20–8. doi: 10.1128/AEM.71.1.20-28.2005
[5] ↑ Best, A., and Abu Kwaik, Y. 2018. Evolution of the arsenal of Legionella pneumophila effectors to modulate protist hosts. MBio 9:e01313–8. doi: 10.1128/mBio.01313-18
