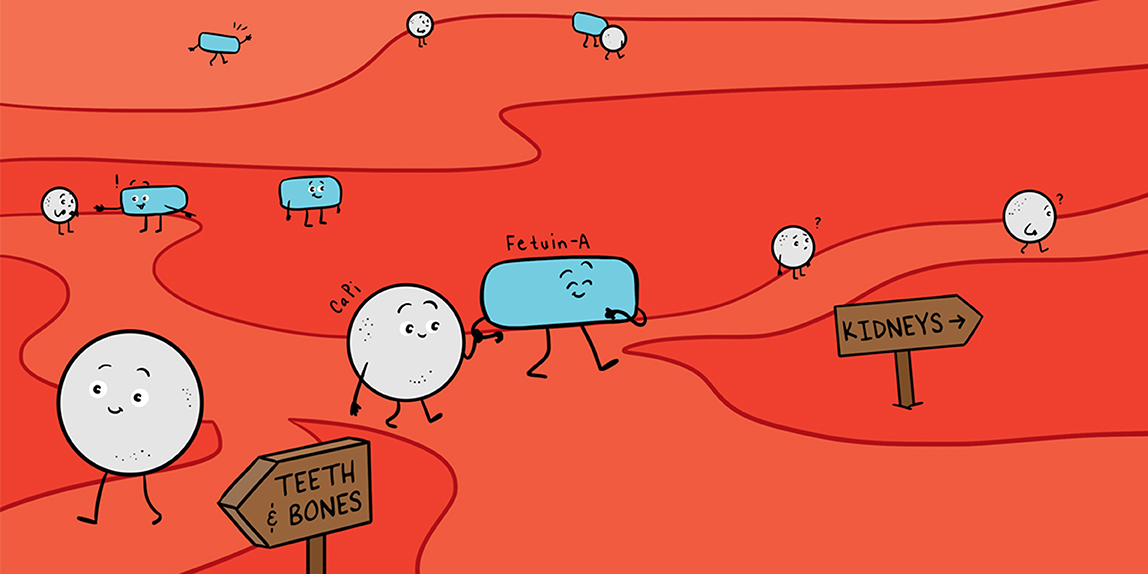
Abstract
Calcium and phosphate are important components of your teeth and bones. Proper incorporation of calcium and phosphate into teeth and bones is called mineralization. If there is too much calcium or phosphate in your blood stream, your body keeps mineralization under control with a protein called fetuin-A. Fetuin-A accompanies calcium and phosphate as they travel through the blood stream, preventing dangerous mineralization from happening in other bodily tissues and helping the excess calcium and phosphate to leave the body through the kidneys.
Hard Facts About Our Teeth
Teeth are special tissues because they become hardened during development. That is good, because then we can use them to chew. The only other hard tissue in the body is the bone. Bones and teeth harden with the help of nutritional building blocks, which we get from the foods we eat. The most important hardening substances are calcium and phosphate. Calcium and phosphate can combine to form a mineral called apatite, which makes bones and teeth hard and strong. The hardening process is called mineralization. If apatite does not form, mineralization does not occur, and our bones and teeth stay soft.
Not all tissues in the body are meant to be hard. Something should protect all soft tissues from hardening like teeth and bones do. Substances responsible for such protection have been discovered, and they are called mineralization inhibitors. The protein fetuin-A is one such mineralization inhibitor—it prevents mineralization from happening in the blood. Too much calcium and phosphate in the blood can form apatite in places where it should not form. A healthy person almost never has too much calcium or phosphate circulating in the blood. The body senses the levels of calcium and phosphate and gets rid of the excess through the kidneys, in the urine.
You often hear: “Drink milk, it is good for your bones and teeth,” and this is true (Figure 1). Children and babies need calcium and phosphate for their growing teeth and bones. Milk is a good source of calcium and phosphate, which is why babies usually get milk or milk porridge every day. It is a good idea to let children drink a glass of milk each day. Calcium-containing foods provide the right amount of calcium for our bodies, but phosphate is a different matter. Ready-made foods, like sodas, often contain a lot of phosphate, which is added because it makes the food look and taste good. Phosphate also extends the shelf-life of food products. If you eat lots of ready-made foods, your body must make extra efforts to get rid of all that phosphate. The kidneys do most of this work.
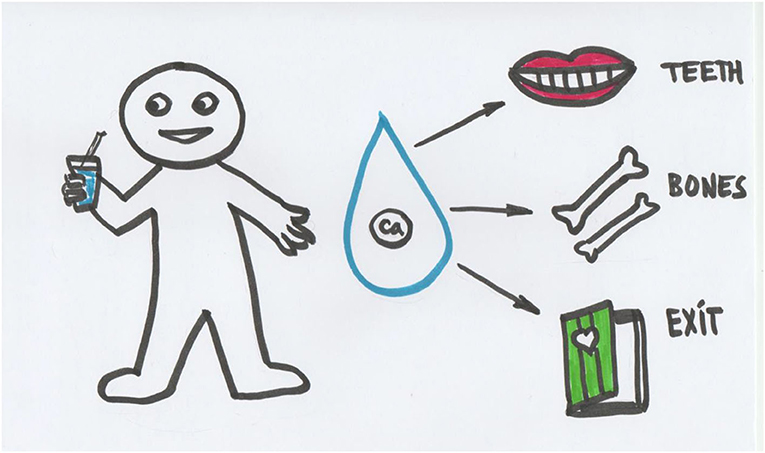
- Figure 1 - Milk contains calcium and phosphate.
- Through digestion, calcium and phosphate enter the blood stream and travel throughout the body. Teeth and bones are one of the important destinations for calcium and phosphate. Excess calcium and phosphate that are not needed for growth are eliminated from the body with the help of kidneys.
Our Teeth As a Construction Site
Your body is built according to a plan encoded in your genes. The construction starts before you are born, and it never stops. Many of the building blocks for this construction come from the food you eat. In your digestive tract, saliva and gastric juices break foods down into smaller components, which serve as building blocks for newly formed tissues. Think about taking apart a Lego® model. Using the same blocks, you can build a new model that could be completely different.
The blood transports the building blocks from food throughout your body, delivering them to the construction sites where growth is happening. Calcium is in high demand by growing bones and teeth. Phosphate and calcium are fetched from the blood stream to construct teeth and bones. They are placed into a soft net of collagen fibers that ultimately shapes your bones and teeth. As soon as the net is filled with building blocks, the tissue becomes hard, like steel-reinforced concrete. Steel and collagen fibers can take a lot of tension. Cement and apatite can take a lot of weight. Thus, both steel-reinforced concrete and bone are tough and flexible at the same time.
Too Much of a Good Thing: Excess Calcium and Phosphate Can Cause Problems
You can provide your body with the necessary building blocks for proper construction through the foods you eat. But you cannot simply ship back excess building blocks if you take in too many! Leftover calcium and phosphate cannot just be stored around construction sites in your body until the next time they are needed—this would disturb the proper functioning of other organs. So, it is a good thing that the body has mechanisms to get rid of the leftovers. These mechanisms switch on before the blood stream becomes too overloaded with excess building blocks. Usually, building blocks circulate in the blood stream until they are used at the construction site. Everything that is not used goes around and around in the blood stream, like a merry-go-round. After a while the body senses that there is to many leftover building blocks and it gets rid of them through the kidney into the urine. When excess calcium and phosphate travel through the body, they connect to some proteins that are also circulating in the blood, to form calcium-phosphate-protein complexes. Too many calcium-phosphate-protein complexes can be harmful. They can settle on soft tissues, such as the walls of blood vessels and these sediments can disturb or even block the flow of blood. Excess calcium and phosphate can also cause the formation of solid pellets of apatite in soft tissues.
For your body to be healthy and function properly, all substances it contains must be in balance. All of the building blocks in your blood stream must be dissolved in the blood. What happens when you try to dissolve too much of a substance in a certain amount of liquid? A simple experiment illustrates the result (Figure 2). Take a glass of water and add salt, spoon by spoon, stirring constantly. At first, the liquid is transparent, but eventually you will add a spoonful that does not dissolve, no matter how vigorously you mix. When you stop stirring, the salt crystals will settle on the bottom of the glass. The solution in your glass is no longer transparent. It is overloaded with salt. The word for this is supersaturated. When a solution is supersaturated, it does not matter how long you stir, no more salt will dissolve. If you hang a thread in the supersaturated solution overnight, in the morning you will find crystals covering the thread. After several days, you will be the happy owner of a beautiful “salt diamond.” This illustrates the fact that only a certain amount of a substance can be dissolved in a given volume of liquid. But what if this happens in your body? No one would like pebbles growing under their skin!
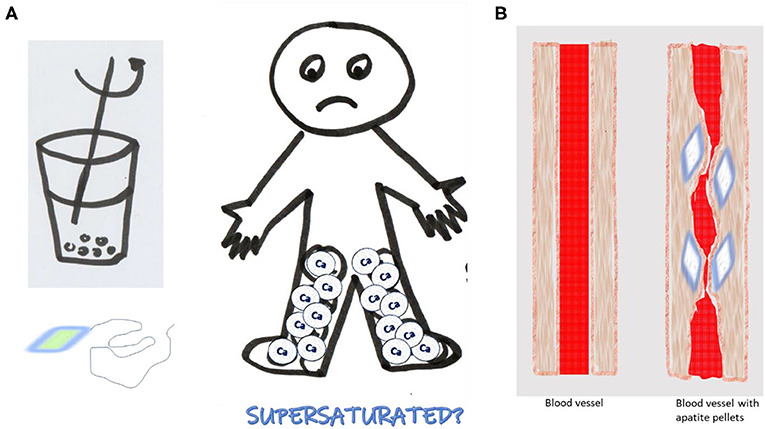
- Figure 2 - (A) If you keep adding spoonful after spoonful of salt to a glass of water, eventually the salt will no longer dissolve, and the solution becomes supersaturated.
- Supersaturated solutions can form crystals. (B) Something similar can happen in the body if excess calcium is not removed from the blood. Uncontrolled calcification can result, in which calcium-phosphate- protein particles settle in soft tissues, like the walls of blood vessels, and can even cause the formation of apatite pellets under the skin, in the walls of blood vessels. Such pellets can make the blood vessels narrow, or completely block the blood flow. Disturbed blood circulation means that the organs get less necessary building materials. It also means that waste products are not removed from the body.
Fetuin-A Protects Us From Calcification Disease
The soft tissues in your body are protected from growing crystals with the help of mineralization inhibitors like fetuin-A. The body produces fetuin-A in the liver. When fetuin-A binds to calcium-phosphate-protein complexes in the blood, it prevents them from settling in the tissues [1] and forming crystals like the salt in the experiment described above (Figure 3). Fetuin-A helps excess calcium and phosphate to leave the body through the kidney into the urine [2].
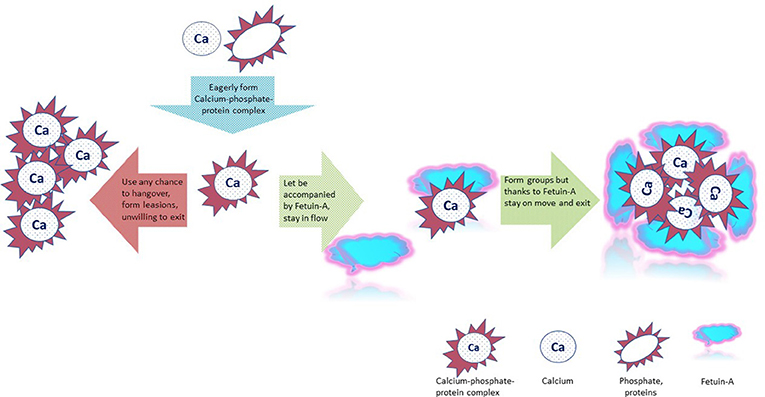
- Figure 3 - Calcium-phosphate- protein complexes normally form in the blood (blue arrow).
- When fetuin-A is present, it binds to these calcium-phosphate- protein complexes and helps to eliminate them from the body (green arrows). When there is no fetuin-A, the calcium-phosphate- protein complexes clump together and can settle in the tissues, causing calcification (red arrow). This is dangerous because crystal formation in muscles and organs can disturb their work and even destroy them. But all this comes later. And this is about how it starts.
You can easily model the ways of calcium-phosphate-protein particles yourself with the help of a handful of thistle flowerheads, a woolen cloth and a piece of aluminum foil. Let some thistle flowerheads roll over woolen cloth. Soon enough they will be stuck anchored due to sharp prickles and tiny hooks on wool fluff. The second portion of thistle flowers will be caught by already fixed thistle heads and form clots. This is the way of the red arrow. Now wrap 3–4 thistle flowers with aluminum foil. Let this newly made shining ball roll over the woolen cloth. It will manage all the way without any problem. This is the way of the green arrows. This shows fetuin-A in action.
So, you can see that the presence of fetuin-A is extremely important! Lack of fetuin-A causes what is called calcification disease, in which tiny mineral pellets develop in the smallest blood vessels. This is dangerous because it can cause crystal formations in muscles, blood vessels, heart, kidneys and even brain. When the tissue which is supposed to be soft and stretchable becomes solid, it cannot function properly. The heart will need more effort for each beat, kidney will need more time for filtering. Heart made of stone cannot contract. The blood vessels encrusted with apatite pellets are not flexible and smooth. They also become narrow or can be clogged. This disturbs the blood flow or even blocks it.
Gone With Fetuin-A
Mineralization of the bones and teeth using the calcium and phosphate from our diets is an important process that helps to harden these bodily structures. However, this process of mineralization must be tightly controlled so that excess calcium and phosphate in the blood stream do not crystalize and cause calcification disease. Luckily, our bodies keep calcification under control. Fetuin-A accompanies calcium-phosphate-protein complexes as they flow through the blood stream and prevents sediments of minerals in soft tissues. Fetuin-A is thus an example of a molecule that protects our bodies from “too much of a good thing”!
Abbreviations
CaPi = Calcium + Phosphate.
Glossary
Minerals: ↑ Minerals are very common in nature. Salt crystals of table salt, diamonds in jewelry, pebbles in the garden are minerals. They had been formed during a long time by accumulation of certain molecules.
Apatite: ↑ Apatite is a mineral containg calcium and phosphate.
Mineralization: ↑ Mineralization is a proper incorporation of calcium and phosphate into teeth and bones, making them hard and strong.
Mineralization Inhibitors: ↑ Substances protecting soft tissues from hardening like teeth and bones do. Mineralization inhibitors can stop mineralization.
Fetuin-A: ↑ is a protein, it prevents mineralization from happening in the blood. Though fetuin-A is a big molecule, we cannot see it with naked eye, nor with magnifying glass, or school microscope.
Collagen Fibers: ↑ Molecules shaped like very long chains, woven together like strings of thread in a rope. In the body, collagen fibers give shape and structure to bones, skin and muscles. They are strong and stretchable.
Supersaturated: ↑ When a solution is supersaturated, no matter how long you stir, no more salt will dissolve.
Calcification: ↑ Calcification disease is a dangerous mineralization in the bodily tissues that are not meant to be hard, such as muscles, blood vessels, heart, brain.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
[1] ↑ Herrmann, M., Babler, A., Moshkova, I., Gremse, F., Kiessling, F., Kusebauch, U., et al. 2020. Lumenal calcification and microvasculopathy in fetuin-A-deficient mice lead to multiple organ morbidity. PLoS ONE 15:e0228503. doi: 10.1371/journal.pone.0228503
[2] ↑ Jahnen-Dechent, W., Büscher, A., Koeppert, S., Heiss, A., Kuro, O. M., and Smith, E. R. 2020. Mud in the blood the role of protein-mineral complexes and extracellular vesicles in biomineralisation and calcification. J. Struct. Biol. 12:107577. doi: 10.1016/j.jsb.2020.107577