Abstract
Within Earth’s surface, there are billions of tons of rocks containing minerals that are reactive with CO2, which is a greenhouse gas that has harmful effects on our planet’s climate. Over thousands of years, these minerals interact with the CO2 in the air, converting the gas into new carbonate minerals and permanently storing the CO2 in a solid form. Today, as society develops solutions to combat the harmful effects of excess CO2 in our atmosphere, the processes carried out by these reactive minerals could present a valuable opportunity. There is a challenge of speeding up the reactions so they happen within days (or even weeks), rather than thousands of years. Climate solutions like this are still new and will require more young and enthusiastic minds to advance their development so our climate is livable for generations to come!
Why is CO2 Harmful, and What Can Be Done?
Since the late 1880s, we have used fossil fuels as our main source of energy. Fossil fuels include coal, petroleum, and natural gas. While they give us a lot of energy, they are also in limited supply, and they emit carbon dioxide (CO2) when we burn them. Release of CO2 into the atmosphere contributes to unfavorable changes in our planet’s climate.
There are several ways to reduce the amount of CO2 being emitted into Earth’s atmosphere. One approach is replacing fossil fuel energy with renewable energy, like solar and wind, which do not emit CO2. Another approach is to use energy-efficient technologies that require less energy, such as electric cars and LED bulbs. However, some CO2 emissions cannot be avoided, and we call these “hard-to-avoid” emissions. These are most often found in industrial processes, like those that produce cement, iron, and steel. Because these products are necessary for building our roads, bridges, and buildings, it is important to find ways to avoid the emissions caused by their production. That is where carbon capture comes in to save the day!
Carbon capture works exactly as it sounds: technologies that “capture” CO2 from emission sources. There are a lot of ways to go about capturing CO2. One approach is called carbon mineralization, in which the CO2 gas reacts with certain minerals to form new minerals, called carbonates.
What Are Carbonate Minerals?
You have likely already seen lots of carbonate minerals since they are the building blocks of things like seashells, chalk, and concrete. These three examples all contain calcium carbonate (CaCO3), which is also called limestone. The idea of carbon mineralization is based on a natural process called mineral weathering, in which certain minerals interact with CO2 in the air, forming white carbonate minerals like those in the White Cliffs of Dover below (Figure 1). This process occurs over thousands of years, but if it could be sped up to take place over hours or days, we could use this approach to capture and store CO2!
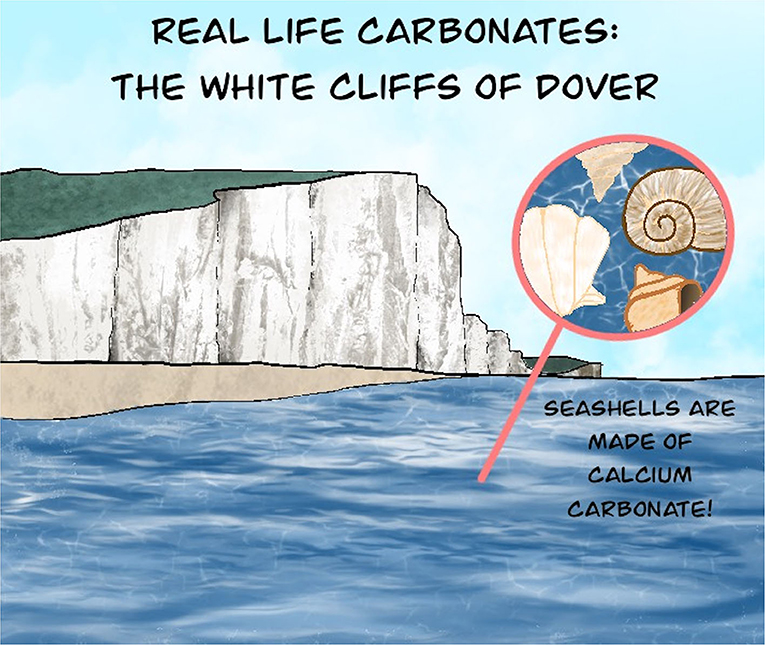
- Figure 1 - You may have already seen carbonate minerals without realizing it.
- Calcium carbonate makes up seashells, chalk, and even the White Cliffs of Dover shown here. Carbonates can form from a variety of ways, all involving interactions with calcium and CO2 that is dissolved in the water.
How Does Carbon Mineralization Work?
The idea of a gas changing to a solid may seem strange, but it happens in other ways that you see every day. The orange rust you see on old metal objects like cars and bikes forms when iron gets wet and reacts with oxygen in the air. The frost you might see on your window in the winter is due to the water vapor in warm humid air touching the cold glass surface. In the case of carbon mineralization, when certain reactive minerals become wet and come into contact with CO2, they react to form carbonates, permanently storing CO2 and keeping it out of the atmosphere for thousands of years.
The reactive minerals used for carbon mineralization need to contain high amounts of calcium or magnesium. These can be found naturally in ultramafic rocks, which are found in the earth on a scale of up to hundreds of billions of tons (>100,000,000,000 tons). Reactive minerals can also be found in wastes from industrial and mining processes. When important materials are made (like iron, steel, or cement) or mined (like diamonds, nickel, or copper), waste is generated and placed in piles near the facility. Much of this waste is rich in calcium or magnesium.
It is important to remember that CO2 is also a waste gas in our atmosphere. Ever since human civilization started using fossil fuels, this heat-trapping gas has been released into the air as a waste product of combustion. Using carbon mineralization, ultramafic rocks and industrial/mining waste could react with the waste CO2 present in the air to form carbonate minerals, helping to remove CO2 from the air and prevent it from accumulating to excessive amounts. This waste management process is illustrated in Figure 2.
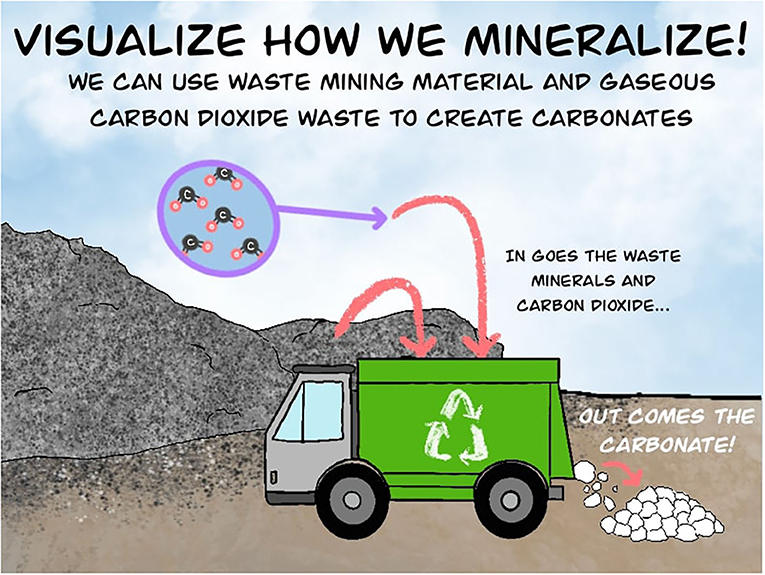
- Figure 2 - Carbon mineralization involves combining two forms of waste to make new carbonate products.
- When valuable materials are made (like iron, steel, and cement) or mined (like diamonds, nickel, and copper), solid wastes are also produced, which are often rich in calcium or magnesium. The same processes that produce or mine these valuable materials often also produce CO2 as a waste that is emitted into the atmosphere. As shown in this figure, the waste CO2 can be combined with the solid wastes to produce new carbonate minerals.
What Can We Do With the Carbonate Products?
The process of using the products produced from carbon mineralization is known as CO2 utilization. Carbon mineralization can be expensive, so by selling the carbonate products, money can be made so that the process is more affordable. Also, if the carbonate products can be made into building materials, like concrete, these new building materials could replace current building materials that emit CO2 when produced, so CO2 utilization could potentially avoid tons of CO2 emissions!
Let us look at concrete as an example. Concrete is made from a mixture of cement, aggregates, and water (Figure 3). Cement acts as the glue that binds the ingredients together. Various sizes of aggregates, such as gravel or sand, are used to provide volume and stability to the concrete. The water allows the concrete to be mixed well before it hardens.
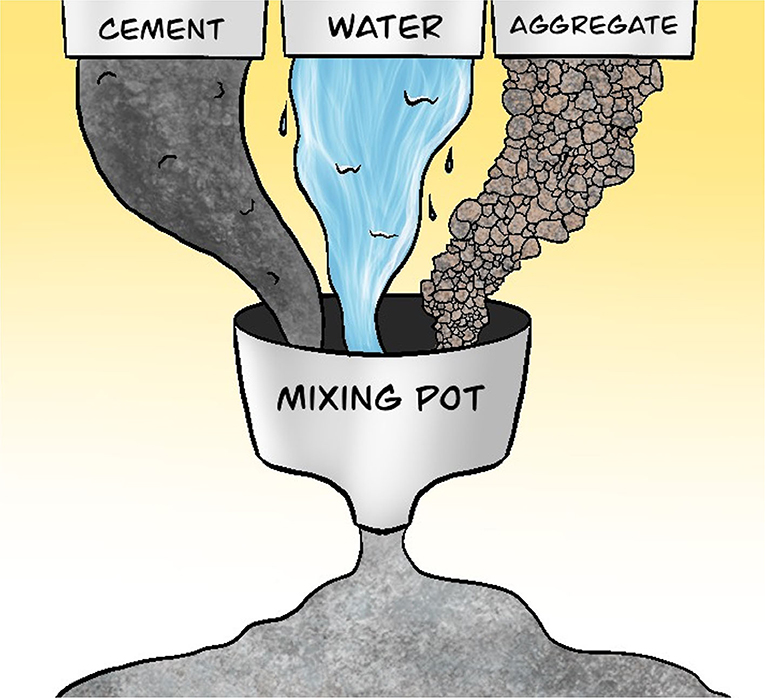
- Figure 3 - Concrete is made up of cement, water, and aggregates.
- Aggregates are particles of various sizes, such as gravel or sand, and they provide volume and stability to the concrete. Cement acts as the glue that binds the aggregates together. Water is added to allow the concrete to mix well before it hardens.
Cement production creates a lot of CO2–it accounts for 8% of all of humanity’s CO2 emissions! Researchers are trying to find ways to reduce cement-related emissions by reducing the amount of cement used in concrete or by changing the way cement is produced. Successful changes in the cement industry could have a major impact on the amount of CO2 being emitted into our atmosphere! Some companies are using carbonate minerals to create new aggregates for concrete that are more useful than normal aggregates. Some companies have figured out a way to inject CO2 directly into wet concrete, where it reacts and causes the concrete to become even stronger once it dries. Other companies get creative by using different sources of calcium (like industrial wastes) as a new cement in their concrete.
There are already companies around the world that are using carbon mineralization to create new building products, but they are facing a difficult task. Most of the building materials we have today are cheap and relatively easy to use, so replacing them requires some creativity. It is not impossible to replace a cheap and useful product with a new one; take phones as an example. Before we had iPhones, we had basic flip phones that were simple, functional, and cheap. The iPhone became popular not because it was more affordable than a flip phone, but because it was a better product. Hence, new carbonate building materials must offer better features than materials that are currently available and release less CO2 during their manufacture than current products do.
How Developed is This Technology?
Building materials made from mineral carbonates have significant potential to decrease CO2 emissions in the cement industry. However, for the technology to become popular, work still needs to be done in several areas:
• Research: The process to make carbonates with CO2 is still expensive and complicated, and it needs to be improved. More research is also needed to prove that the new cement and concrete are better than the ones we have now.
• Carbon incentives: There are ways for the government to pay money to people who store CO2 and prevent it from entering the air, “incentivizing” CO2 storage and use. This would help carbon mineralization be more affordable.
• Regulations: There are strict regulations that dictate which materials can be used to make concrete for roads and buildings. Some of the regulations are old and could be updated to encourage the use of new carbonate materials. This would depend on whether the new materials are proven to perform well.
Carbon Mineralization in the Ocean
Just as CO2 is floating in our air, it is also dissolved into our oceans. This is similar to how CO2 is dissolved in your fizzy soft drinks. The CO2 gas likes to be in equilibrium between the air and water. Because there is more CO2 in a soda can than in the air around it, when you open a soda can, the CO2 quickly leaves the can and enters the air so it can reach equilibrium.
Our oceans do not make bubbles like soda because the CO2 is already in equilibrium between the ocean and the air. By adding calcium and magnesium to the ocean, the CO2 dissolved in the ocean can mineralize and form carbonate minerals, reducing amount of CO2 in the water and disturbing the equilibrium. This will cause more CO2 to leave the air and enter the ocean. Scientists are researching safe ways to add calcium and magnesium to the oceans so that we can use the help of the oceans to remove CO2 from the atmosphere! [1].
How Can You Get Involved?
Carbon mineralization is just one of the many solutions that are needed to stop Earth’s climate from changing. All of the solutions will require help from all sorts of minds. For example, while scientists and engineers can help to develop carbon mineralization and other technologies, politicians and lawyers can help with government policies that encourage climate solutions, and journalists can communicate new technologies to the public. Additionally, we can all try to reduce our own carbon emissions by changing our everyday habits, such as by using less electricity or carpooling. Climate change will be a big problem for many years to come, but if everyone takes their own steps to help the fight, the problem could very well be solved!
Glossary
Renewable Energy: ↑ Energy that comes from a source that is in such abundance that it cannot be used up, such as wind or solar energies.
Carbon Capture: ↑ A technology that captures CO2 from emission sources (or directly from the air) to prevent the CO2 from causing changes to Earth’s climate.
Carbonates: ↑ Solid minerals that are formed from interactions with CO2 and water.
Mineral Weathering: ↑ A natural process where rocks and minerals break down to form new rocks and minerals. Weathering can involve reactions with CO2 from the atmosphere, leading to carbonate formation.
Ultramafic Rock: ↑ Rocks with relatively large amounts of magnesium, making them ideal for carbon mineralization.
CO2 Utilization: ↑ The process of changing CO2 into a new form and using it as a valuable product.
Aggregates: ↑ Non-reactive materials that provide volume and stability to concrete, ranging from small sand particles to larger gravel rocks.
Equilibrium: ↑ A balance between opposite forces.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
[1] ↑ Renforth, P., and Henderson, G. 2017. Assessing ocean alkalinity for carbon sequestration. Rev. Geophys. 55:636–74. doi: 10.1002/2016RG000533
