Abstract
Physics is the human attempt to discover the unchanging laws of the universe. Since the Greek thinkers 2,000 years ago, every now and then there have been changes in the physical laws that scientists once thought were unchangeable. These are times when humans discover a new “land,” in which unexpected phenomena challenge and contradict current thinking. About 120 years ago, one of the biggest of these changes revealed a new and exciting land—a land that we are still exploring today. In this article, I will describe some of the strange phenomena of this new land, called quantum physics, and how it forced us to change the way we think about the world in terms of energy, scientific measurements, and the reality of the objects around us. As no one—even the best physicists in the world—knows exactly what it really means, I will leave it to you to decide!
Quantum Theory: The Beginning
What do creative physicists do when confronted with phenomena that do not match what they already know? They may try to “stretch” an existing theory so that it fits the new phenomena, or they may boldly offer a completely new point of view. Sir Isaac Newton, the English physicist and mathematician of the seventeenth century, did exactly that when he proposed a world view that was completely different from that of Aristotle, who lived in Greece 2,000 years ago and is considered the father of modern science.
At the end of the nineteenth century, two physicists, Max Planck and Albert Einstein, proposed a new and surprising way to view several unexplained phenomena, one of which is called the photoelectric effect. In doing so, Planck and Einstein came up with an idea that marked the beginning of a new and revolutionary theory—quantum theory. This theory, which grew step by step, was completely different from anything physicists had ever thought about—it was a revolution in science.
What Is The Photoelectric Effect?
But what exactly is the photoelectric effect? In short, it has to do with the way light can generate electricity (Figure 1). If blue light falls on a conductive metal like copper, sometimes the metal ejects electrons, which are tiny, negatively charged particles that circle around the much bigger atomic nucleus, which is positively charged. The movement of electrons creates an electric current that can be measured and be used. The color of light is important—if red light is used instead of blue light, no electrons are ejected and no electrical current is created. This phenomenon has been known since 1887, based on experiments done by Heinrich Hertz, a German physicist.
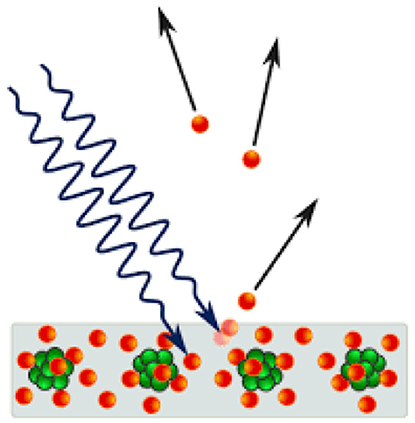
- Figure 1 - The photoelectric effect.
- When blue light (squiggly black arrows) hits a conductive metal like copper, it knocks some of the negatively charged electrons (red balls) away from the positively charged nuclei of the copper atoms (green balls). Under suitable conditions, these electrons leave the metal and create an electric current. This effect is only possible because light consists of tiny packets of energy with no mass, called photons. The photons of other colors of light, such as red light, do not contain enough energy to knock the electrons free.
The big problem with the photoelectric effect was that, at that time, light was considered to be a wave (like ocean waves). According to the wave theory of light, if more and more waves of red light were used, this should eventually make the waves big enough to eject electrons and create an electric current. So why does this not happen with red light, even though it happens even with very weak blue light? Einstein figured this out in 1905. Instead of referring to light as waves, Einstein suggested that we should look at light as little “packets” of energy, but with no mass at all. Packets of blue light have much more energy than packets of red light, which is why they cause the photoelectric effect. Think of this as tiny billiard balls of energy—if a ball hits an electron strongly enough, the electron may be ejected from the metal. This strange idea successfully explained the photoelectric effect, and the tiny charged light packets were named photons, which is the Greek name for light. Things did not end with this astonishing new idea—in fact, it was just the beginning. Within a few years, a huge number of new and unusual phenomena were discovered. It was a whole new world, with new laws! Newton’s orderly and predictable world, which resembled a huge machine made up of many small parts, was replaced by an unexplored land, full of surprises [1]! Physicists did not discard Newton’s laws; those laws could still explain and predict many phenomena, like the movement of the moon around the Earth, or a stone falling to the ground. However, beneath this world, a vast, new one had been discovered.
The Mystery Of The Quantum Jump
As you have probably learned, every substance is made up of countless atoms. Atoms are like tiny solar systems that cannot be seen, even with a very powerful microscope. In these tiny solar systems, the electrons are the planets, which orbit around the nucleus (the sun). Physicists discovered that electrons can move from an orbit close to the nucleus to an orbit further away from it, and vice versa, if the electrons gain or lose energy. The wonder of the quantum world is that those tiny planets (the electrons) move from orbit to orbit in a single jump. They are always in one particular orbit—either close to the nucleus or farther from it—but never halfway, a third of the way, or a quarter of the way in between. For example, an electron can be close to the nucleus of an atom and can absorb energy from a photon of light. Then, at the very next moment, it is in a more distant orbit. This phenomenon is called the quantum jump (Figure 2).
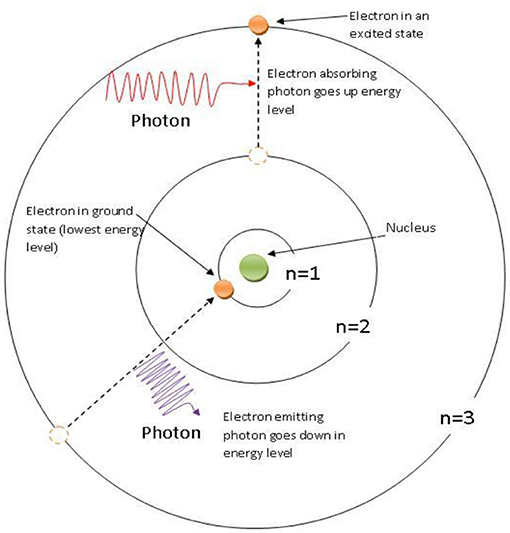
- Figure 2 - A quantum jump of an electron in a hydrogen atom.
- The nucleus of the atom is indicated by the green dot. Two electrons (orange dots), have been traveling around the nucleus in orbits called n = 1 and n = 3. The one in n = 1 absorbs a photon of light and gains the energy to make a quantum jump—in the blink of an eye—to n = 3, which is further from the nucleus. The electron in n = 3 emits a photon and drops down to an orbit closer to the nucleus. At no time is either electron seen to exist anywhere between orbits 1 and 3, and this phenomenon has still not been explained by scientists!
How does the electron get from one orbit to another? As of today, no one has a good answer. This is one of the great mysteries of quantum theory: what happens to the electron during the jump from orbit to orbit? Does it “die” in one orbit, to be “born” again in a different orbit? Does it move so fast that our devices cannot track it? Maybe you can think of a creative idea to solve this mystery.
Telepathy Between Pairs Of Particles
Do you believe in mind reading, also called mental telepathy? Physicists tend to be very rational and logical people, and the vast majority of them think that mental telepathy between people is an unscientific, irrational, and illogical phenomenon that probably does not happen.
But in the new land of quantum theory, particles in a special experimental system seem to have a kind of telepathy! Imagine we have two particles located far apart from each other—let us say a kilometer or even much more—and we measure a certain property of one of the particles, such as its speed. Countless experiments have shown that, for some properties, the value of the measured property of one particle will always be the “mirror image” of the property of the other particle. If we think of the two particles as people, this would mean that every time one person raised their right hand, the other person would raise their left hand, and vice versa—even though neither of them can see which hand the other is raising [2]! Many experiments have shown that one particle in these pairs somehow influences the other—without any prior coordination, and even at a great distance. This very strange and special phenomenon is called quantum entanglement.
You may be wondering if every single pair of microscopic particles is in a state of quantum entanglement. The answer is no—this only happens in pairs of particles that were initially in a special connected state (like twins), called a singlet state. Furthermore, quantum entanglement is very sensitive and fragile; if any external particles enter into the experimental system, the entanglement is destroyed—so the experimental system must be completely isolated from the outside world.
Currently, a tremendous and expensive effort is being made to build a quantum computer, which is a type of computer based on the phenomenon of quantum entanglement. Such a computer must be completely isolated from any outside disturbances that could destroy the quantum entanglement.
Mysterious Questions About Cats
Another mystery of the quantum world was investigated by the Austrian physicist Erwin Schrödinger. Schrödinger described a very strange quantum situation. According to the mathematics of quantum theory that Schrödinger himself helped to develop, a quantum particle is found in all its possible states at the same time. For example, if you had a set of quantum dice and threw one of them, while it is in the air its state will be all of the possible results—a little bit 1, and a little bit 2, and a little bit 3, and 4, and 5, and 6! When you actually check the result, it will show only one possibility. The condition before the dice hit the table is called superposition. According to this theory, a quantum particle only “chooses” one state when it is observed. This might sound crazy, but it sounds even crazier if we think about it in terms of the world we know. Schrödinger came up with a thought experiment involving a cat inside a closed box. In the box with the cat there is one radioactive atom that has a 50% chance of decaying and triggering a hammer that breaks a vial with poison, which kills the cat. But there is 50% chance that the radioactive atom will not decay and the cat will stay alive. According to quantum theory, the cat would be both alive and dead at the same time—only when we open the box to look does it actually become one or the other (Figure 3).
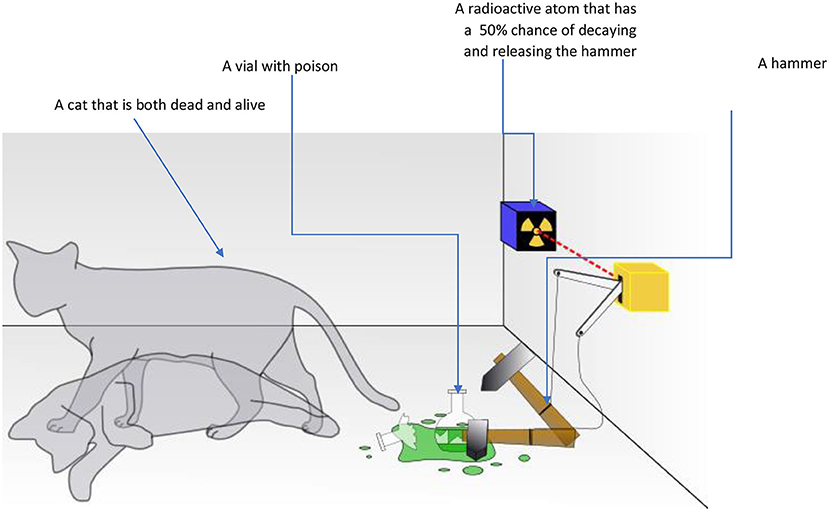
- Figure 3 - In Schrödinger’s thought experiment, a sealed box contains a cat, a vial of poison, and a radioactive atom that has a 50% chance of breaking down during the experiment.
- If the atom breaks down, the poison vial will be shattered, and the cat will die. If the atom does not break down, the cat remains alive. According to the mathematics of quantum theory, the cat would be simultaneously alive and dead until someone opened the box to look—at that point, one of the states becomes reality.
How can this be? What was the state of the cat when the box was closed, and no one was looking at it? Was the cat both alive and dead? Did we cause it to be one or the other, just by looking? Currently, quantum theory still does not provide a satisfactory explanation.
The Uncertain World Of Physics
Another spectacular quantum phenomenon was formulated by a German physicist named Werner Heisenberg, and it is known as the Heisenberg uncertainty principle. Heisenberg stated that there are special quantum states in which if we know, for example, the exact speed of an electron, we lose all knowledge of its location. According to this principle, the opposite is also true—if we know exactly where an electron is, we lose all ability to know its speed! But, if we know approximately what the speed of the electron is, we can know approximately what its location is [1].
In general, measurement in the quantum world is strange and completely different from the way people thought about measurement using classical physics. In the old “land,” we could photograph a falling stone, and the photograph would not affect the stone in the slightest—it would continue to fall as if nothing had happened. Not so in this new land! When photographing an electron, the photograph significantly affects the location or the speed of the electron! Physicists still cannot explain this fascinating effect. Does the measuring always change the thing being measured? If so, what was the electron before we measured it? Maybe it was something completely different, and only the fact that we measured it caused it to “become” an electron.
Physicists like to ask a strange question in the context of quantum theory: if a tree falls in the forest and no one hears it, does it make a sound? The answer to this question may seem obvious, but when you think about quantum land, it is not so obvious after all—it is another version of the cat-in-the-box question: what was the state of the cat before we opened the box [3]?
What Does It All Mean?
I have given you only a small taste of the world of quantum phenomena, and I leave you with a question: what does this strange new land mean? It would appear that we have no way to know what is happening in a quantum system when we are not looking at it. We can only calculate the probability that something will happen (like the probability that the cat will be alive when we open the box, vs. the probability that it will be dead). When I was studying physics at university more than 30 years ago, one of my professors said something like this: quantum theory is like a chef cooking with a microwave—he knows how to use it, but has no idea how it works.
Some physicists believe that all the possible outcomes of a quantum experiment occur at the same time, but in separate, distinct universes. According to this concept, we are in one of these universes and are only aware of the one we are in, but there are copies of us in all the other possible universes! For example, in Schrödinger’s thought experiment, if I found the cat to be alive, there is another universe—one that I have no connection to and know nothing about—in which I see the cat is dead.
Others claim that it is the human brain that creates all the quantum strangeness. Proponents of this idea think that quantum oddities would not exist in a world without humans. Remember the question I asked earlier: if a tree falls in the forest and no one is around to hear it fall, does it make a sound? Those who hold this approach will say “no,” because they believe that humans are the center of the physical universe. Albert Einstein did not like this idea, and once asked his friend, “Do you really believe that the moon does not exist when you are not looking at it?”
Others, myself among them, think that if the quantum world describes a reality where everything is connected—not separate and divided as we usually perceive it—then quantum phenomena suddenly seem natural and logical. According to this approach, there is a new type of wave that exists throughout space that instantly connects all its parts and particles. For example, this wave instantly connects two entangled particles, as well as other, larger “particles,” such as the moon and the Earth. The cat in Schrödinger’s experiment would have a wave that exist in two superposition states, live and dead, but the cat himself would be all the time in only one state, dead or alive with 50% chance for each state. What will define his final state depend on his initial state which is unknown precisely to us.
Which approach would you pick?
Glossary
Photoelectric Effect: ↑ When a certain type of light hits atoms, the air becomes charged with electricity. In 1905, Einstein explained this using new principles that laid the foundation for quantum theory.
Quantum Theory: ↑ A new theory of physics that began to develop in the beginning of the twentieth century, and tries to explain how the tiny parts that make up atoms work.
Quantum Jump: ↑ Electrons seem to “jump” from one orbit (or energy level) around the nucleus to another, without existing anywhere in between.
Quantum Entanglement: ↑ Mysterious connections between two microscopic “twin” particles that might be far away from each other. Entangled particles behave like they have telepathic connection, faster than the speed of light.
Superposition: ↑ A quantum situation in which two opposite possibilities exist at the same time but, when we observe them, we see only one of the two possibilities.
Heisenberg Uncertainty Principle: ↑ A quantum principle stating that we cannot know both the position and speed of a tiny particle—the more we know about one property, the less we know about the other.
Probability: ↑ A way to measure how likely something is to happen. For example, when we observe for an hour a radioactive atom, we can only predict the chances that it will decay in an hour.
Conflict of Interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
[1] ↑ Bohm, D. 1989. Quantum Theory. Mineola, NY: Dover Publications Inc..
[2] ↑ Sakurai, J. J. 1985. Modern Quantum Mechanics. Boston, MA: Addison-Wesley Company, Inc. p. 223–32.
[3] ↑ Bell, J. S. 1986. “Six possible worlds of quantum mechanics,” in Proceedings of the Noble Symposium 65: Possible worlds in Arts and Sciences. Stockholm.