Abstract
As an organism develops from a single fertilized egg cell, how do all the different organs and tissues of its body come to be? How do some cells know to become nerves, others skin, and others bone or blood? We have spent decades studying a protein called Notch that plays a role in these kinds of decisions. Notch sits on the cell surface and helps cells communicate with each other to decide whether to grow, divide, specialize, or stay quiet. This communication, called signaling, guides how cells organize themselves during development. In this article, we describe how Notch carries messages from the outside of a cell all the way to the nucleus, where genes are turned on or off. Our work in flies and worms helped show that this system is important in many animals, including humans. Understanding how Notch works has revealed fascinating connections between development, normal tissue upkeep, and even diseases like cancer and Alzheimer’s.
Drs. Artavanis-Tsakonas, Greenwald, and Struhl were awarded the 2025 Canada Gairdner International Award “For pioneering work on the Notch signalling pathway, which has significantly contributed to our understanding of how cells communicate with each other during development, how these signals regulate cell fate determination and how disruption can lead to developmental defect and cancer”.
Decisions, Decisions
All animals are made of millions (or even billions) of cells, arranged in astonishingly complex and carefully repeated patterns. Have you ever looked closely at a butterfly wing? Butterflies of the same species have wings that are nearly identical, with the same shapes, sizes, and colorful patterns of stripes and spots. To build such precise and beautiful patterns, cells need to know where they are, decide what to do, and coordinate their choices with their neighbors.
But how do cells know what to become? In the earliest stages of life, the first few cells that make up an embryo are almost the same. But as the body takes shape, those cells start to make decisions. One might become part of the nervous system. Another might help form an eye, or a digestive organ, or a limb. These decisions depend on conversations between cells—signals that say things like, “You become a nerve cell—I will become a skin cell” or “You stay quiet while I divide”. These cell-to-cell conversations happen constantly as the body takes shape, ensuring that every part develops at the right time and in exactly the right way. One of the most important ways this happens is through a communication system called Notch signaling.
The three of us have spent many years uncovering how Notch signaling works, from identifying the gene, to the first “handshake” between two cells, all the way to the changes inside the cell’s control center, the nucleus. We each focused on different aspects of the process, often using different organisms. Our discoveries, along with those of many other researchers, have helped explain one of the most important communication systems in biology. Iva was fascinated by this problem from a young age. “As a child, I had a book on the human body and was amazed that it all starts with one cell”, she said. “So, I was curious about ‘developmental biology’ even before I knew there was such a field”.
Meet Notch: A Protein That Helps Cells Communicate
The gene that encodes the Notch protein was first identified more than 100 years ago, when scientists studying fruit flies found a mutant fly with notched wings (Figure 1). But it was not until the 1980s that the gene (DNA instructions) that caused the wing defect when mutated was identified [1]. Around the same time, a similar gene called LIN-12 was discovered in C. elegans, a microscopic roundworm, and shown to code for a protein that is the worm version of fly Notch [2]. Finding that Notch-related proteins help cells communicate in both flies and worms—two very different species—suggested that this signaling system is likely to work in a similar way across many species—even humans. In addition, the ability to study these proteins in both systems allowed researchers to take advantage of the distinct experimental strengths of each organism.
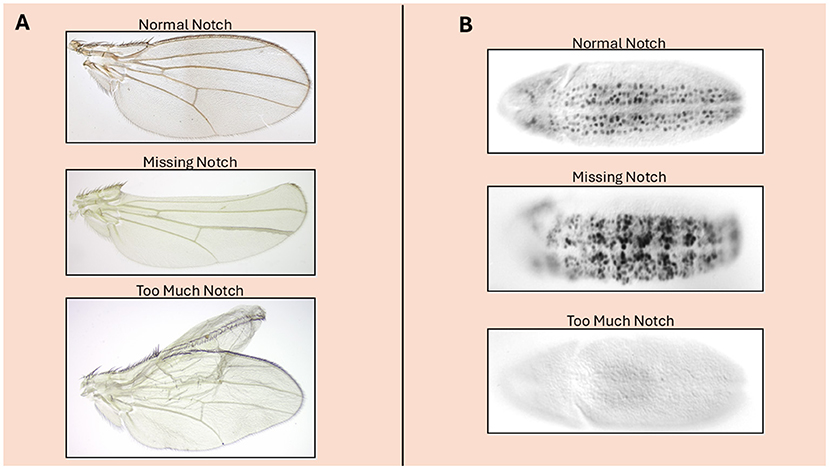
- Figure 1 - (A) A fruit fly wing.
- When Notch is “missing” from part of the wing, some wing tissue is lost (creating a “notch”). Conversely, cells with “too much” Notch can induce an extra wing. (B) When Notch is functioning normally in a fruit fly embryo, cells called neuroblasts (which will develop into nerve cells, stained black) develop in an organized pattern because cells communicate via Notch so that some cells become skin cells while others become neuroblasts. When Notch is missing, no cells get the message, and all become neuroblasts. When there is “too much” Notch, all cells become skin cells, and none become neuroblasts.
Notch sits on the surface of many cells, with one part outside the cell and another part inside (Figure 2A). You can think of it like a sensor: it helps a cell respond to signals from its neighbors and decide what to do next. The signal comes from a partner protein, called a ligand, on the surface of a neighboring cell. For the signal to work, the two cells must touch to bring Notch and its ligand together, like a handshake (Figure 2B). These signals happen again and again during development to help cells decide whether to divide, specialize, or just chill out. One special feature about how Notch works is that it is often involved in communication that goes both ways, from one cell to its neighbors and then back again, allowing cells to coordinate their choices about what to do.
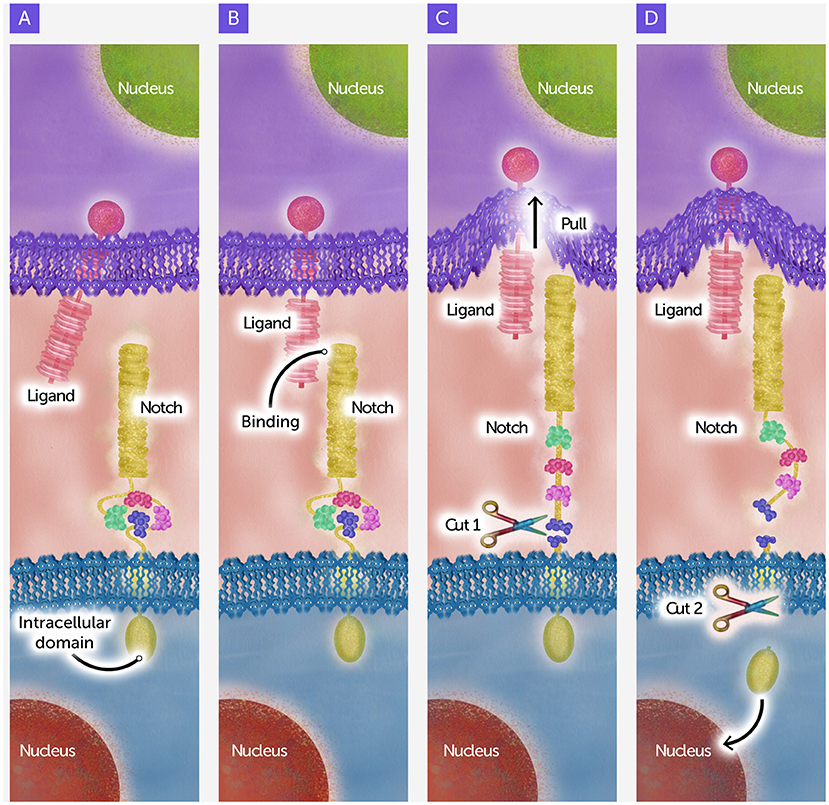
- Figure 2 - Events in Notch signaling.
- (A) Notch and its ligand on neighboring cells. (B) Notch and its ligand bind, as if the molecules are “shaking hands”. (C) The signal-sending cell pulls the ligand back into itself, through a process called endocytosis. This stretches out a special part of Notch that sits just outside the cell, exposing a site that is cut by a first “molecular scissors” called Kuzbanian. (D) This cut allows the rest of the protein to be cut by a second molecular scissors called Presenilin. The second cut releases the intracellular domain so it can travel to the nucleus to control the activity of development-related genes (Figure credit: Somersault18:24).
Cutting the Signal Loose
Once researchers realized that Notch helps cells make decisions, the next big question was: how does a signal on the outside of a cell control how decisions are made by turning genes on or off inside the nucleus? The answer turned out to be both surprising and elegant.
When Notch “shakes hands” with its ligand, special molecular “scissors” cut the protein in two places—first outside the cell membrane and then inside (Figures 2C, D). These cuts release a portion of Notch within the cell, called the intracellular domain, which can then enter the nucleus. This finding raised the possibility that the intracellular domain might travel to the nucleus to control the activity of development-related genes—but this “cleavage and nuclear import” hypothesis needed proof.
To get this proof, new molecular tools were developed in fruit flies to track whether the intracellular domain of Notch could get to the nucleus and, once there, turn genes on and off (Figure 2D) [3]. Further studies in worms and flies confirmed the hypothesis by identifying proteins called Kuzbanian and Presenilin as the molecular scissors that make the first and second cuts, respectively, releasing the intracellular domain for its trip to the nucleus [2, 4]. Overall, this work helped explain how a signal that begins at the surface of a cell can change what happens deep inside.
Pull to Activate
At first, many scientists thought that the ligand handshake was enough to activate Notch—but there was another surprise in store. Instead of just touching, the signal needs to tug on Notch (Figure 2C) [5]. After the ligand binds to Notch, the signal-sending cell pulls the ligand back into itself, through a process called endocytosis. Imagine it like the signaling cell yanking its hand back during the handshake. This yank unfolds a portion of Notch on the outside of the cell, causing it to be cut by Kuzbanian, the first molecular scissors. It is only after this first cut happens that Presenilin, the second molecular scissors, can do the final cut that releases the intracellular domain. So, without the tug, neither of the cuts happen.
One of the first clues about the tug came from studies showing that special “adapter” proteins are needed in the sending cell to bind the ligand and mark it for endocytosis [5]. The process of endocytosis then stretches the Notch molecule and exposes the right part of Notch to the Kuzbanian scissors. “The idea that Notch needs to be yanked open to be activated was a big advance”, Gary explained. “What brought it to our attention was finding the specific adapter proteins necessary for the pull”.
Notch in Health and Disease
Notch is not just important for helping embryos develop correctly. Even in adult organisms, Notch allows cells to make choices based on signals from their neighbors. This process helps regulate cell division and repairs normal wear-and-tear on tissues, especially tissues that are constantly growing or changing like the skin or intestines. It even helps brain cells communicate with each other and form memories of past experiences.
When the Notch pathway is too active or not active enough, it can garble the communication between cells and cause them to make the wrong decisions [6]. This can cause problems in development; for example, some heart defects that babies are born with are associated with mutations in Notch. Many other molecules also help control the activity of Notch. They make sure the signal is not too strong or too weak, which is important for healthy development and for preventing disease. For example, a gene Iva found in C. elegans that prevents “too much” Notch signaling can help stop certain blood cells from growing out of control in a type of cancer called leukemia [6]. Spyros has discovered how these additional layers of control add complexity to the basic Notch signaling system [7], findings that he says “make us realize how closely connected different biological systems can be”.
Looking Back, Thinking Ahead
Together, our work has helped uncover how Notch signaling allows cells to communicate in precise, coordinated ways—from the moment cells begin “shaking hands” to the changes in gene activity deep inside the nucleus. Notch is essential during development and continues to shape the body throughout life. Although this research began with simple organisms like fruit flies and worms, the discoveries turned out to be important for many animals, including humans. These discoveries helped reveal how changes in a single pathway can affect everything from development to disease.
Famous scientists are often asked what they would say to young people who are curious about science or want to help others. For Spyros, it starts with persistence and a sense of wonder. “I never had a moment when I considered quitting”, he said. “When you analyze biological phenomena, there is always a next step and a next question. Have I been disappointed by some experiments? Certainly. But in doing science, this is part—a big part—of our existence”. Iva reminds young scientists that progress is rarely neat—there are always confusing results, failed experiments, and dead ends. But if you stay with it, the moment something finally works can be unforgettable. Gary emphasizes that science is not just about collecting facts—it is about thinking clearly, building strong arguments, and having the courage to question what others take for granted. In a time when scientific evidence is sometimes dismissed, misunderstood, or bent to fit someone’s opinion, we find hope in your generation. Young people who are curious, think critically, follow the evidence, and keep asking bold questions even in the face of challenges are the ones who will carry science—and society—forward.
Glossary
Notch: ↑ A protein found on many cells that helps them “talk” to their neighbors and decide what to do during development, repair, and other important processes.
Signaling: ↑ The way cells send and receive messages, often using special proteins, to help them make decisions, work together, or respond to changes in the body.
LIN-12: ↑ A gene in roundworms that encodes a protein closely related to Notch that helps cells decide what to become during development.
Ligand: ↑ A molecule on the surface of one cell that binds to a receptor on another cell—like Notch—to start a signal between the two.
Intracellular Domain: ↑ The part of a protein, like Notch, that is inside the cell. When released, it can travel to the nucleus and help control which genes are active.
Kuzbanian: ↑ The first “molecular scissors” that helps cut Notch after it receives a signal. This first cut prepares Notch for the second cut by Presenilin.
Presenilin: ↑ The second “molecular scissors” that helps cut Notch after it receives a signal. This cut releases a piece that goes to the nucleus and turns genes on or off.
Endocytosis: ↑ A process where a cell pulls molecules on its surface back inside by wrapping them up in membrane—a key event in activating Notch signaling.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We wish to thank Dr. Susan Debad for providing us with an excellent first draft and continued collaborative input as co-author as well as her thoughtful questions. IG and GS are both funded by grants from the Institute of General Medicine of the National Institutes of Health, R35GM131746 to IG and R35GM127141 to GS.
AI tool statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
References
[1] ↑ Wharton, K. A., Johansen, K. M., Xu, T., and Artavanis-Tsakonas, S. 1985. Nucleotide sequence from the neurogenic locus Notch implies a gene product which shares homology with proteins containing EGF-like repeats. Cell 43, 567–581. doi: 10.1016/0092-8674(85)90229-6
[2] ↑ Greenwald, I. 2012. Notch and the awesome power of genetics. Genetics 191, 655–669. doi: 10.1534/genetics.112.141812
[3] ↑ Struhl, G., and Adachi, A. 1998. Nuclear access and action of Notch in vivo. Cell 93, 649–660. doi: 10.1016/S0092-8674(00)81193-9
[4] ↑ Struhl, G., and Greenwald, I. 1999. Presenilin is required for activity and nuclear access of Notch in Drosophila. Nat. 398, 522–525. doi: 10.1038/19091
[5] ↑ Langridge, P., and Struhl, G. 2017. Epsin-dependent ligand endocytosis activates Notch by force. Cell 171, 1383–1396. doi: 10.1016/j.cell.2017.10.048
[6] ↑ Vlierberghe, P. V., and Ferrando, A. 2012. The molecular basis of T cell acute lymphoblastic leukemia. J. Clin. Invest. 122, 3398–3406. doi: 10.1172/JCI61269
[7] ↑ Guruharsha K, G., Kankel M, W., and Artavanis-Tsakonas, S. 2012. The Notch signalling system: recent insights into the complexity of a conserved pathway. Nat. Rev. Genet. 13, 654–666. doi: 10.1038/nrg3272