Abstract
How do you sense temperature and pain? Our ability to detect heat, cold, pain, and the spiciness of “hot” peppers relies mainly on TRP, a group of channel proteins first discovered in the fruit fly eye. This was followed by the discovery of the TRPV1 channel in humans, which responds to heat and capsaicin, the spicy ingredient in “hot” peppers. These findings facilitated the identification of additional TRP channels involved in sensing temperature and pain, thus significantly advancing our understanding of sensory perception. In this article, we describe a boy with an insensitivity to “hot” peppers. Genetic analysis revealed that he carries a rare mutation in TRPV1, rendering the channel non-functional and explaining his lack of sensitivity to “hot” peppers. This unique case provides valuable insight into the molecular basis of temperature and pain sensing and highlights how genetic variations in sensory proteins can shape individual experiences of the environment.
How Do We Feel Painful Temperature?
Every day, when you take a shower, you adjust the temperature of the running water to feel comfortable. Sometimes, when the water temperature is too hot and painful, you must add cold water to make it cooler. Likewise, when the water is too cold and unpleasant, you add warm water. How do people sense heat and cold? When and why do the warm or cool sensations become painful? What exactly is the sensation of pain? (see Nerves, pain and consciousness, Frontiers for Young Minds). Many researchers worldwide deal with these questions. In 2021, David Julius received the Nobel Prize in Medicine in recognition of his discovery of channel proteins that are opened (activated) by temperature, among them the TRPV1 protein (read more in Hot Chili Peppers Help Uncover the Secrets of Pain, Frontiers for Young Minds) [1]. This channel plays a key role in the sensation of some types of pain and “opens” in response to several stimuli: temperatures higher than 42°C (the temperature that causes pain), an environment that is very acidic, or the application of a substance called capsaicin, found in “hot” peppers. The discovery of TRPV1 helped researchers discover other proteins that are activated by different temperatures and increased their understanding of the mechanisms of temperature and pain sensations [2]. In this article, we will present a boy who likes to eat “hot” peppers and learn how he contributed to our understanding of temperature sensing [3].
What is Genetic Screening, And What is it Good For?
One way to figure out how something works is to look at what happens when it breaks down. Imagine that you want to understand how a car works. A car has many parts—wheels, steering wheel, engine, brakes, and more. If you take out one part at a time and see what breaks down, you can understand that part’s function. For example, if you take out the steering wheel, the car will not be able to turn. If you take out the engine, it will not be able to move. But, you could also check the function of the various parts in another way. For example, if you want to find out which part is responsible for stopping the car, you could randomly remove parts, one at a time, and see when the car fails to stop. In this way, you would discover that the important parts for stopping are the brake pedal, the wire connecting the pedal to the brake, the brake plate, and the brake fluid.
In biological research, scientists use similar methods to understand the role of the various proteins in the body, of which there are at least 20,000. To discover the function of a particular protein, researchers induce mutations in experimental organisms (like bacteria, cells, plants, and animals) to disrupt protein’s function and examine the bodily changes caused by these mutations. Alternatively, if researchers want to understand which proteins participate in vision, they can induce random mutations in proteins and study the cases in which the mutations cause vision problems. Then, the mutated proteins can be located and identified (see The transcription of life: From DNA to RNA, Frontiers for Young Minds). This method is called genetic screening, and it helps researchers identify new proteins - some of which were never suspected to be involved - in specific biological processes, such as sensory perception. It allows scientists to better understand how the body functions in health and disease, and how certain conditions might be treated or cured. Sometimes, nature creates mutations randomly. It is not ethical for researchers to induce mutations in humans to see what happens, but occasionally we discover humans with random mutations (usually because they have a disease or another medical problem) who agree to undergo simple, harmless tests to help researchers understand their conditions.
A Mutation Eliminating the Sensitivity to “Hot” Pepper
One day, a young boy found a bowl of “hot” peppers that his mother had collected to make a stew, and he started eating them. His mother noticed something strange: the child did not panic, flinch, cry, or yell like other children who eat “hot” peppers—in fact, he did not seem to feel the spiciness of the peppers at all. The surprised mother decided to take the child for a medical examination. The doctors found that, although the child was completely healthy, he did not feel the burning sensation that normally arises when eating “hot” peppers. To understand why, the doctors read scientific articles dealing with sensitivity to spiciness. They found that the channel protein TRPV1 mediates the feeling of spiciness, and it is activated (opened) by capsaicin. Then, they examined the gene encoding for this protein in the child and discovered that the child carried a rare, previously unknown mutation in TRPV1 (Figure 1).
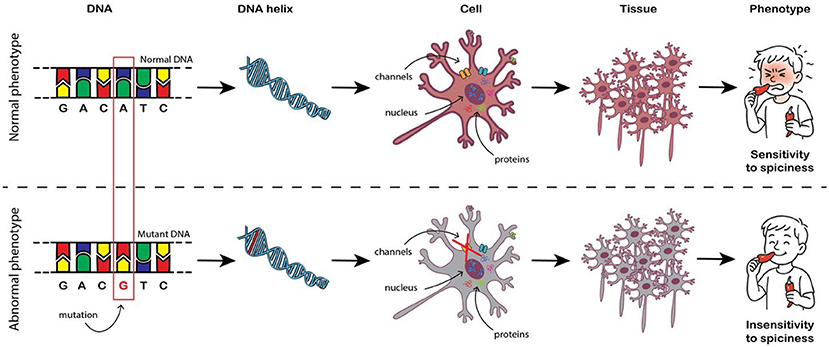
- Figure 1 - How a mutation in DNA can cause disease?
- The body’s tissues are made up of cells. Each cell contains, among other components, proteins that play important roles. Proteins are produced according to “instructions” found in DNA, which is located in the cell’s nucleus. Changes in the sequence of DNA, called mutations, can change the “instructions” for producing proteins. The change may cause the protein to operate differently, which may lead to a change in the function of the cell or tissue and could cause a genetic disease.
Experiments on Isolated Cells
To understand how the child’s gene mutation affects the activity of the TRPV1 protein, experiments were carried out on human cells that can be grown outside the body for a long time. These cells, which produce TRPV1, were divided into two groups: one group contained cells that produced the normal TRPV1 protein (without the child’s mutation), and the other contained cells that produced the defective protein (with the child’s mutation). We then examined how the cells with and without the mutation responded to capsaicin. As expected, when we added capsaicin to cells with the normal TRPV1 protein, they showed a normal response. In contrast, no response was observed in cells with the mutant protein.
This observation told us that the mutation causes a loss of TRPV1 activity. There are several possible explanations for this result. First, the protein with the mutation might not be made in the cells at all. Second, the protein with the mutation might end up in a different place in the cell, where it cannot generate a response to capsaicin. The third possibility is that the protein, which is made up of 4 identical parts, is not assembled properly. All these possibilities were examined and ruled out: that is, the protein with the mutation is produced, located, and assembled properly in the cell! Thus, the only remaining possibility is that the mutation probably prevents the activation of TRPV1 by capsaicin.
The TRPV1 Mutation Modifies Temperature and Pain Sensing
TRPV1 belongs to a protein group found in special nerve cells that respond to tissue-damaging stimuli and produce the sensation of pain. TRPV1 is activated by painful heat, high acidity, and capsaicin. TRPV1 also plays a role in the persistent pain felt during injury and inflammation, and in the process that regulates body temperature. Despite all this information, mostly from lab experiments, a major mystery remains: What is the function of TRPV1 in humans? It is likely that it works similarly in humans and animals, but are we sure? Maybe it plays roles found only in humans, or it has roles that have yet to be discovered in animals.
The child and his family agreed to participate in a series of examinations in which a stimulus (such as heat, cold, or capsaicin) is applied, and the subject reports: what he feels (hot, cold, or pain), the extent of the sensation (almost nothing, mild, or strong), and when he feels it. We were particularly interested in how the TRPV1 mutation affected whether the child would feel hot and cold temperatures like the rest of us, and if he developed inflammation like other people. A computer-controlled metal probe was attached to the child’s skin to apply precise temperature changes. Then, we asked the child to report by pushing a button, both when he felt heat or cold sensations and when he began feeling pain in the hot or cold temperature ranges.
The results showed normal heat and cold sensitivity (Figures 2A, C), but decreased sensitivity to painful heat, namely, the child reported a sensation of hot pain at a higher temperature compared to his family members and healthy volunteers (Figure 2B). This result is similar to previous results seen in mice that lacked TRPV1 [4, 5]. The child’s results in response to cold pain were surprising: he reported a sensation of cold pain at a much higher temperature compared to his family members and healthy volunteers (Figure 2D), meaning he was very sensitive to cold temperatures. TRPV1 is known to be activated by hot, not cold temperatures, and later studies in mice without TRPV1 also showed that the mice had a high sensitivity to painful cold—an interesting result!
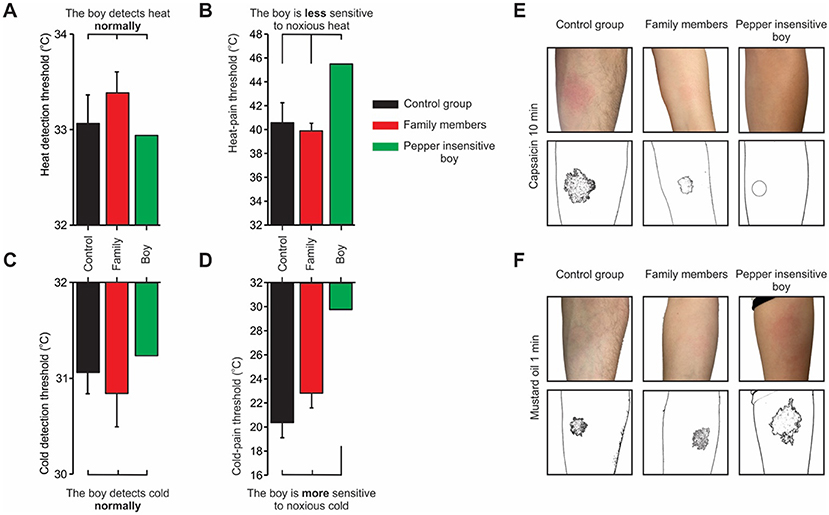
- Figure 2 - (A, C) The child with the TRPV1 mutation has a normal sensitivity to heat and cold compared to healthy subjects.
- (B) However, the child (green bar) is less sensitive to painful heat, and (D) more sensitive to painful cold compared to his family members (red) and healthy volunteers (black). The child’s family and healthy volunteers showed an inflammatory response (redness) on the skin following the application of (E) capsaicin or (F) mustard oil. In contrast, the child did not develop an inflammatory response to capsaicin but developed an enhanced inflammatory response to mustard oil. Illustrations below the photographs show the size of the inflammatory response.
Application of capsaicin to the skin normally causes local inflammation, with redness and mild pain. In our experiments, this effect was observed in the child’s family members and in healthy volunteers. However, when we applied capsaicin to the child’s skin, it had no effect (Figure 2E). There are two main possibilities to explain this result: an insensitivity of the child’s skin to capsaicin or the inability of the child’s skin to develop inflammation. To test the first option, the child was given capsaicin orally. As expected, he did not feel any burning sensation. In contrast, his family members and healthy volunteers strongly felt the spiciness of the capsaicin. To test the child’s ability to develop an inflammatory response on the skin, we applied mustard oil, which also causes local inflammation and mild pain. To our surprise, the mustard oil caused a stronger inflammatory response on the child’s skin compared to his family members and healthy volunteers (Figure 2F) [3].
In conclusion, our results suggested that the child’s TRPV1 mutation prevented activation of the protein. The child showed low sensitivity to painful heat, high sensitivity to painful cold, and high sensitivity to mustard oil on the skin (Figure 3). Thus, this study sheds new light on the mechanisms of temperature and pain sensations.
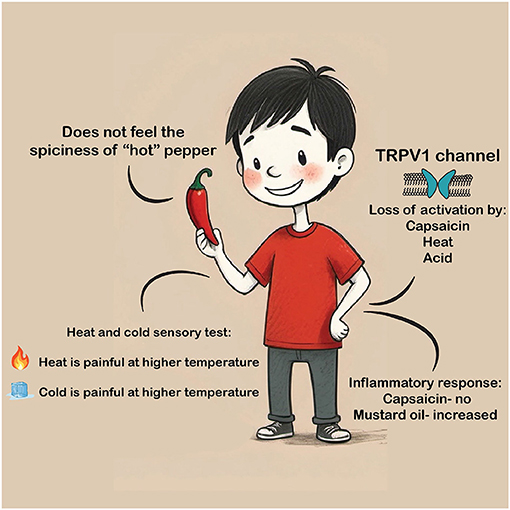
- Figure 3 - Our study led to the following insights: The TRPV1 mutation prevents it from working properly.
- The lack of TRPV1 activity causes insensitivity to “hot” pepper in the mouth, the absence of an inflammatory response following application of capsaicin to the skin, increased skin inflammatory response to mustard oil, low sensitivity to painful heat, and high sensitivity to painful cold.
Looking to the Future
Pain is a protective mechanism that helps us avoid harm, but when pain becomes chronic, it can severely affect quality of life. Millions of people suffer from chronic pain conditions that are poorly understood and difficult to treat. Understanding the molecular basis of pain—like how TRPV1 and other proteins work—can pave the way for more effective and targeted treatments. This unique case of a child with a TRPV1 mutation offers new insight into how humans sense temperature and pain. Notably, the child’s extreme sensitivity to cold and mustard oil points to biological mechanisms that are still not fully understood. These findings may lead scientists to explore alternative pain pathways and open the door to new areas of research, especially into cold-induced pain and inflammatory responses. By studying rare mutations in people, researchers can uncover how specific proteins contribute to sensory perception and pain, knowledge that can eventually lead to the development of new painkillers or treatments for chronic conditions, offering relief to those in need.
Glossary
Channel Protein: ↑ A type of protein in cell membranes that forms a tunnel, allowing specific molecules or ions to pass in and out of the cell.
TRPV1: ↑ A channel protein that senses heat, acidity, and capsaicin- the "hot" ingredient of chili peppers. It helps the body detect painful heat and causes the burning feeling from spicy food or inflammation.
Capsaicin: ↑ A compound in “hot” peppers that triggers a burning, spicy sensation by activating TRPV1, the channel protein that senses heat and pain.
Mutation: ↑ A change in the DNA sequence that can alter how a protein is made. This may affect how the body works and sometimes leads to diseases.
Genetic Screening: ↑ A research method where scientists induce random mutations in genes to see how they affect the body. It helps reveal what different proteins do and how diseases happen.
Gene: ↑ Segment of DNA that codes for specific proteins or traits, such as eye color. Genes are inherited from parents and often provide the instruction for cells to produce proteins.
Inflammation: ↑ A natural response of the body to injury or infection, causing redness, swelling, and sometimes pain. It helps the body heal, but too much inflammation can be harmful.
Chronic Pain: ↑ Long-lasting pain that continues for months or years, often after an injury has healed. It can affect daily life and may not respond well to typical treatments.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This research was supported by the Israel Science Foundation (ISF) (Grant no: 593/24).
Original Source Article
↑Katz, B., Zaguri, R., Edvardson, S., Maayan, C., Elpeleg, O., Lev, S., et al. 2023. Nociception and pain in humans lacking a functional TRPV1 channel. J. Clin. Invest. 133:e153558. doi: 10.1172/JCI153558
References
[1] ↑ Caterina, M. J., Schumacher, M. A., Tominaga, M., Rosen, T. A., Levine, J. D., and Julius, D. 1997. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389:816–24. doi: 10.1038/39807
[2] ↑ Rosenbaum, T., Morales-Lázaro, S. L., and Islas, L. D. 2022. TRP channels: a journey towards a molecular understanding of pain. Nat. Rev. Neurosci. 23:596–610. doi: 10.1038/s41583-022-00611-7
[3] ↑ Katz, B., Zaguri, R., Edvardson, S., Maayan, C., Elpeleg, O., Lev, S., et al. 2023. Nociception and pain in humans lacking a functional TRPV1 channel. J. Clin. Invest. 133:e153558. doi: 10.1172/JCI153558
[4] ↑ Caterina, M. J., Leffler, A., Malmberg, A. B., Martin, W. J., Trafton, J., Petersen-Zeitz, K. R., et al. 2000. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science 288:306–13. doi: 10.1126/science.288.5464.306
[5] ↑ Davis, J. B., Gray, J., Gunthorpe, M. J., Hatcher, J. P., Davey, P. T., Overend, P., et al. 2000. Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature 405:183–7. doi: 10.1038/35012076
