Abstract
Mycobacteria are ancient types of bacteria that can cause serious diseases in both people and animals. The best-known are Mycobacterium tuberculosis, which causes a lung disease called tuberculosis, and Mycobacterium leprae, which causes a disease of the skin and nerves called leprosy. Mycobacteria can spread through the air, through contaminated food or water, or on dirty surfaces. Mycobacteria have clever ways to avoid the body’s defenses, so it is very hard to treat these infections. Patients must take strong medicines called antibiotics for many months. But some mycobacteria have become resistant, meaning they are no longer killed by antibiotics. In this article, we explain what scientists have learned about the tricks mycobacteria use to escape from the body’s defenses and how they cause disease. With this knowledge, scientists hope to find new treatments so that infections with antibiotic-resistant mycobacteria can be cured in the future.
What are Mycobacteria?
Mycobacteria are a fascinating type of bacteria believed to have originated more than 150 million years ago. Mycobacteria grow very slowly. It can take some mycobacteria a day to double from one cell into two—something other bacteria can do in minutes or hours. Another thing that makes them stand out from other bacteria is their thick, waxy cell wall, which is why they are called “myco”, meaning waxy.
There are more than 200 species of mycobacteria, and about one in ten are known to cause disease in humans (Figure 1). Mycobacterium tuberculosis causes the disease tuberculosis, which mostly hurts the lungs and can make people cough a lot, have chest pain, feel hot with fever, lose weight, and feel very tired. Another member of the mycobacteria family, Mycobacterium leprae, causes leprosy, a disease that damages the nerves in the skin so badly that people may, for example, lose fingers or toes. Other mycobacteria mainly infect animals. For example, Mycobacterium bovis causes tuberculosis in cows but can also spread to humans. Finally, another major group of mycobacteria, called non-tuberculous mycobacteria, includes Mycobacterium avium and Mycobacterium marinum. Some of these non-tuberculous mycobacteria cause disease, while others are harmless or are dangerous only to people with weak bodily defense systems, like those who already have another serious disease.
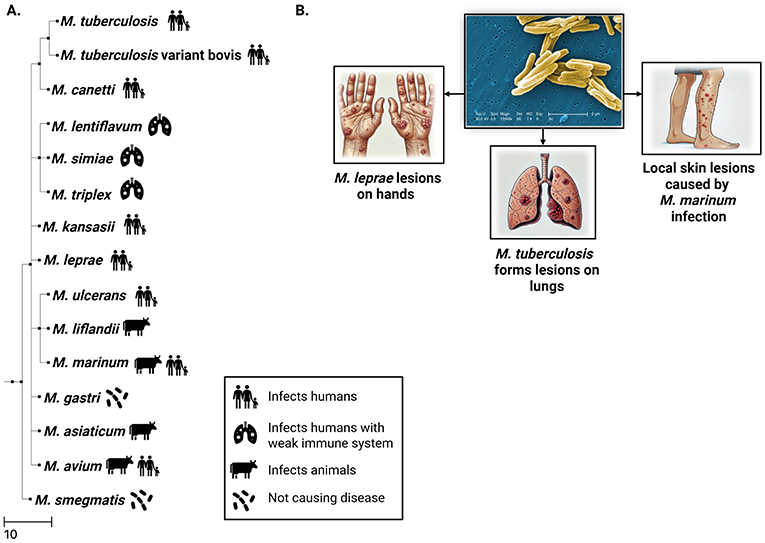
- Figure 1 - Mycobacteria are a group containing around 195 species.
- Although most mycobacteria do not cause disease, a few species can infect humans and animals. (A) Family tree showing how disease-causing mycobacteria are related and which humans or animals they infect. This tree shows which mycobacteria are more similar to each other and which ones changed a long time ago during evolution. (B) Mycobacteria are rod shaped with a thick, waxy cell wall. Infections with some types of mycobacteria can cause damaged spots called lesions on the skin, lungs, or other places in the body.
Mycobacterial Diseases: Past and Present
In the years between 1600 and 1800, there was an outbreak of tuberculosis in Europe and the United States. At that time, the disease was called the “Great White Plague” due to the extreme paleness of infected people, or “consumption” because the disease caused a lot of weight loss, which looked like the illness was slowly consuming these people or eating them up.
At that time, no medicines were available, and people with tuberculosis were sent to special places called sanatoriums where they could get rest and were “treated” with sunlight and fresh air. Similarly, people with leprosy were forced to live in so-called leper colonies, away from healthy people. One such colony was located on the Greek island Spinalonga, which only closed in 1957. Today, tuberculosis is less common in Europe and the United States, but it is still a huge problem, especially in Africa and Asia. Around 10 million people become ill with tuberculosis each year and more than one million die from it. Non-tuberculous mycobacteria are also worrisome, as infections with these types occur more often nowadays and are very difficult to treat.
How Can We Treat Diseases Caused by Mycobacteria?
You will remember that the COVID-19 pandemic came to an end when a Vaccine against the coronavirus was developed. A vaccine trains the immune system to fight the infection so well that the disease has no chance anymore. Scientists have been working for many years to find a vaccine that can end the tuberculosis pandemic, but so far they have not done so. The only vaccine that helps a little bit to prevent tuberculosis is more than 100 years old. This vaccine (called the BCG vaccine) helps protect children against the most severe forms of tuberculosis, like when tuberculosis spreads to the brain, but it does not work very well for the kind that affects the lungs.
It was not until 1943 that the first antibiotic against tuberculosis was discovered. Unlike a vaccine, which prevents the disease, an antibiotic cures the disease because it stops the bacteria from growing or kills them. The problem is that mycobacteria can sometimes change so that the Antibiotics do not to work on them anymore. In other words, the mycobacteria resist the antibiotics, so this is called Antibiotic-resistant. Because of antibiotic resistance, it is important that scientists find new medicines to treat mycobacterial infections.
How do Mycobacteria Infect Us?
One of the most common ways mycobacteria can infect us is through the air. When someone who has tuberculosis coughs or sneezes, for example, this can produce tiny droplets containing mycobacteria, which are released into the air. If another person happens to be close by, they can inhale these tiny droplets and get infected (Figure 2). Another way to get infected is through food or water contaminated with mycobacteria. Fortunately, food and water are very safe in most places, so people do not get sick this way very often. Some mycobacteria, like M. marinum, can also cause skin infections. These bacteria can enter a person’s body if they have cuts or wounds and touch places where these bacteria grow. This can happen when people have a pet fish aquarium at home, in which M. marinum likes to grow.
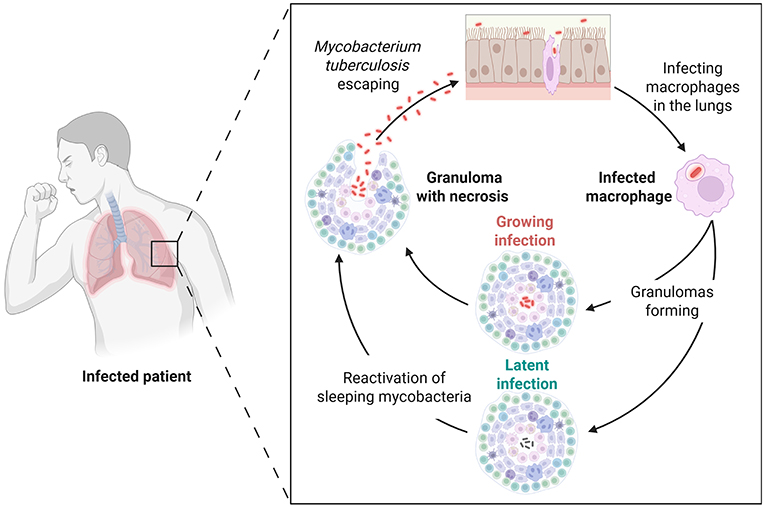
- Figure 2 - M. tuberculosis infection begins with breathing in mycobacteria spread when another patient sneezes.
- Mycobacteria are taken up by lung macrophages, and trick the macrophage’s defense systems. Eventually the infected macrophages die. New macrophages come to fight the bacteria, forming a lump of immune cells called a granuloma. The granuloma is like a prison where mycobacteria can live for a long time. As long as the immune system is strong enough, the granuloma keeps the patient safe from the mycobacteria, which are in a “sleeping” state. If the immune system weakens, mycobacteria “wake up”, break out of the granuloma, and spread through the body, continuing the cycle.
What Happens During Infection?
Our bodies are very good at fighting diseases and other dangers and keeping us healthy. The first line of defense includes body parts that stop bacteria from getting in, like the skin or the mucus and hair in the nose. If mycobacteria still manage to sneak in, the body’s second line of defense, the Innate immune system, is turned on. The innate immune system fights mycobacteria with white blood cells, which come in several types. One type is called macrophages, which stands for “big eaters” because these cells “eat up” bacteria or other germs. Once the mycobacteria enter macrophages, they are trapped inside tiny bubbles called Phagosome [1]. Eventually, the phagosome joins with another tiny bubble, known as a Lysosome (Figure 3A). The lysosome is filled with chemicals that can break down everything inside, leaving nothing but waste. The macrophage then shows bits of waste to other immune cells, and that is the sign for the Adaptive immune system, the third line of defense, to kick in. Adaptive immunity uses other types of immune cells (namely T cells and B cells) to fight the infection and make sure the body remembers it, so that the person does not get sick again from the same thing.
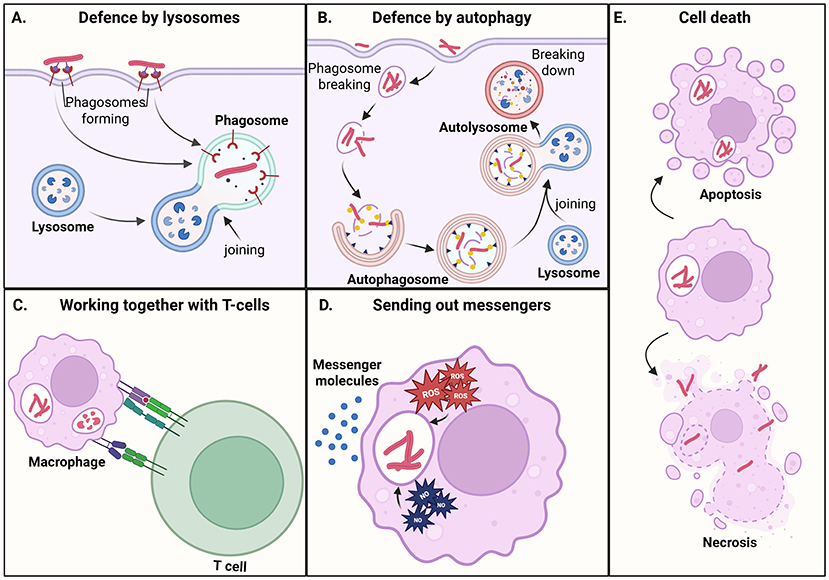
- Figure 3 - Macrophages can defend themselves against mycobacteria in several ways: (A) Phagosomes containing mycobacteria join with lysosomes that break down the bacteria.
- (B) Mycobacteria that escape the phagosome end up in an autophagosomes, which also destroys them. (C) T-cells (from the adaptive immune system) send out messengers telling macrophages to turn on their defenses. (D) Macrophages send messengers to other cells, to attract more immune cells and help them make defense weapons (ROS and NO in the figure). (E) Macrophages can kill themselves by apoptosis, preventing mycobacteria from growing. Mycobacteria can also cause necrosis, in which macrophages burst open and release the bacteria, helping them spread.
The Clever Ways Mycobacteria Avoid Being Destroyed
Mycobacteria have special ways to hide from the body’s defenses. Once mycobacteria are inside a macrophage, they begin planning their escape. For instance, they can avoid being destroyed by blocking the fusion between phagosomes and lysosomes, shutting down the cell’s breakdown system. Additionally, some types (like M. tuberculosis and M. marinum) produce a protein that damages the phagosome so they can escape from this tiny balloon and move into the cytoplasm [2, 3]. In the cytoplasm, mycobacteria get more nutrients that help them grow.
If mycobacteria manage to escape, this sets off an alarm within the macrophage. The escaped bacteria are marked by proteins that help the macrophage attack them using another defense system called autophagy. Autophagy is a system our cells use to keep themselves healthy and clean. Think of it this way: if you do not clean your room every so often, you may start to collect old clothes, trash, broken toys, and so on. Similarly, cells accumulate things that are not useful anymore, like old proteins or damaged cell parts. Autophagy can also help clean up intruders like mycobacteria. When mycobacteria are captured this way, they get trapped inside little bubbles called autophagosomes (Figure 3B), which deliver the bacteria to the lysosomes for destruction. Yet, some mycobacteria can escape from this defense system too.
Dying Cells: Foe and Friend of Mycobacteria
Mycobacteria can cause macrophages to die and use the dead cells as “food” that helps them grow faster (Figure 3). But the body has smart ways to fight back, including a special kind of cell death called apoptosis (Figure 3E). Apoptosis is a planned way for a cell to break down itself into tiny pieces. It helps the body stay healthy and get rid of infections without causing too much swelling or damage to nearby cells.
Necrosis is a different kind of cell death, which is not as good for the body. During necrosis, a cell swells up until it bursts and lets out all its contents. Necrosis can be switched on by mycobacteria. When cells burst, a lot of proteins get out and this causes inflammation. Inflammation can make a person feel sick with swelling, pain, and fever. When inflammation happens, a lot of macrophages are attracted to the dying cells. These macrophages also become infected because they take up the mycobacteria that escaped from the dying cells. If this goes on for a while, lots of white blood cells pile up into little lumps around the infected macrophages (Figure 2). These lumps of cells are called Granuloma. Granulomas explain how tuberculosis got its name: “tuberculum” in Latin stands for “little lump”. Granulomas can grow to a few millimeters or a centimeter—big enough to be seen on X-rays. Infected macrophages within the granuloma often die from necrosis, releasing nutrients that help the mycobacteria grow.
When cells keep getting infected and dying again and again, mycobacteria can spread to nearby cells or even into the blood. This can be very dangerous and might damage the body’s tissues and organs. Scientists have learned that a granuloma is like a double-edged sword. On one hand, it is like a prison wall built around mycobacteria to keep them away from the rest of the body. At the same time, the granuloma helps mycobacteria to move from one cell to another by causing some cells to die.
A Glimpse Into the Future of Treating Mycobacterial Infections
About nine out of 10 people who become infected with M. tuberculosis (or other mycobacteria) will not develop any disease symptoms at first. Some people can kill off the mycobacteria, but most people infected with M. tuberculosis develop a “sleeping” form of tuberculosis, in which the bacteria are not doing anything or are growing very slowly. If the immune system is not strong enough, the mycobacteria start growing more rapidly and people may get severely ill. Old age, other illnesses, certain medicines, or traits people are born with can all cause a weakened immune system that cannot protect against tuberculosis or other mycobacterial diseases.
Mycobacterial diseases can be cured by antibiotics, which also prevents the spread of the infection to other people. However, mycobacteria have a thick wall around them that does not let antibiotics go through easily [4]. The ability of mycobacteria to hide inside cells makes it even harder for antibiotics to get close to them. This explains why patients only get better if they take more than one antibiotic, sometimes 3–4 at the same time. Patients also need to take antibiotics for at least six months, and sometimes over a year. It is very hard for patients to keep taking medicines for so long, but if they stop too early, mycobacteria can survive and change over time, becoming antibiotic resistant. When this happens, it becomes very difficult to find another way to cure the patient.
Scientists and doctors are working very hard to find new ways to help patients so that mycobacterial diseases can be cured faster. One possibility is to give antibiotics together with medicines that give the patient’s immune system a boost, making it better at fighting mycobacteria [5]. For example, some medicines help reduce inflammation in tuberculosis patients. This is very important because inflammation causes painful, dangerous swelling in tissues around the heart or brain. These same medicines also save infected macrophages from necrosis, the harmful kind of cell death that helps mycobacteria spread to new cells.
Sometimes, medicines made for other diseases can also help fight mycobacterial infections. Scientists are already looking at medicines used to treat problems that often affect older people, like diabetes, or problems with the heart and blood vessels. These medicines can work by stimulating autophagy, which helps macrophages clean up mycobacteria. Researchers worldwide keep studying mycobacteria and all their clever ways to hide in human cells. Hopefully, this will soon result in new ways to treat patients, both with antibiotics and with immune-boosting medicines, so that tuberculosis will eventually be a disease of the past.
Glossary
Vaccine: ↑ A medicine that prepares the immune system to recognize and fight specific infections later—without causing illness—so the body can respond faster and more effectively if exposed again.
Antibiotics: ↑ Medicines that fight bacterial infections by killing bacteria or stopping their growth. They do not work against viruses and should only be used when prescribed for bacterial illnesses.
Antibiotic Resistance: ↑ Bacteria can undergo changes so they become resistant, meaning that antibiotics can no longer kill them or stop them growing.
Innate Immune System: ↑ The first response the immune system shows toward an infection. White blood cells like macrophages “eat up” mycobacteria, with the aim to destroy them.
Phagosomes: ↑ A tiny bubble inside a white blood cell that forms when the cell “eats up” germs like bacteria to break them down.
Lysosome: ↑ A tiny bubble inside a cell filled with harmful chemicals. When lysosomes join with phagosomes these chemicals help breaking down the bacteria.
Adaptive Immune System: ↑ A more specific defense that kicks in after the innate immune system. It learns to recognize and fight mycobacteria using special types of cells like T cells and B cells.
Granulomas: ↑ A small lump formed by white blood cells that surrounds and contains a mycobacterium infection.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This publication is part of the project “Live or let die: the intracellular fate of pathogenic mycobacteria” with file number OCENW.XL21.XL21.006 of the research programme ENW-XL which is financed by the Dutch Research Council (NWO). Figures 1–3 were made with biorender.com and an open AI software, DALL-E, was used to draw the pictures of lesions in Figure 1.
AI Tool Statement
The author(s) declare that no Gen AI was used in the creation of this manuscript except for drawing the pictures of lesions in Figure 1.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us
References
[1] ↑ Rohde, K., Yates, R. M., Purdy, G. E., and Russell, D. G. 2007. Mycobacterium tuberculosis and the environment within the phagosome. Immunol. Rev. 219:37–54. doi: 10.1111/j.1600-065X.2007.00547.x
[2] ↑ Abdallah, A. M., Gey van Pittius, N. C., DiGiuseppe Champion, P. A., Cox, J., Luirink, J., Vandenbroucke-Grauls, C. M., et al. 2007. Type VII secretion—mycobacteria show the way. Nat. Rev. Microbiol. 5:883–91. doi: 10.1038/nrmicro1773
[3] ↑ Simeone, R., Sayes, F., Lawarée, E., and Brosch, R. 2021. Breaching the phagosome, the case of the tuberculosis agent. Cell. Microbiol. 23:e13344. doi: 10.1111/cmi.13344
[4] ↑ Saxena, S., Spaink, H. P., and Forn-Cuní, G. 2021. Drug resistance in nontuberculous mycobacteria: mechanisms and models. Biology. 10:96. doi: 10.3390/biology10020096
[5] ↑ Kilinç, G., Saris, A., Ottenhoff, T. H. M., and Haks, M. C. 2021. Host-directed therapy to combat mycobacterial infections. Immunol. Rev. 301:62–83. doi: 10.1111/imr.12951