Abstract
Have you ever noticed how many metallic objects you use every day? Everything, from your jewelery or phone to the cars you ride in and the bridges you cross, contains metal! Metals are shiny materials, but if they are left in the air, they gradually become damaged. For iron and steel, this damage is called rust, scientifically known as corrosion. To protect metallic objects from damage, we must protect them from corrosion. We do this by applying a protective coating that shield the metal, a bit like the sunscreen you apply before going outside. Paint is a protective coating (think of fire hydrant). But if we create corrosion in a controlled way on light metals, like aluminum, it can actually create a robust protective coating! This process is called anodizing. We just need a battery, a container, and Coca-Cola. Welcome to the fascinating world of electrochemistry!
Watch an interview with the authors of this article to learn even more! (Video 1)
- Video 1 - This video showcases the Authors’ talk given live at the Élargis Tes Horizons/Expand Your Horizons event in Switzerland to the Young Reviewers present in person.
- They explain: Metals are essential in daily life, but can corrode, which shortens their lifespan. While corrosion is usually prevented, light metals can be protected by intentionally corroding their surfaces in a controlled process called anodizing. Anodizing is quite simple! We just need a battery, a container and an appropriate liquid, which can be as simple as Coca-Cola or lemon juice. In this video, we will explore anodizing and the fascinating properties of this coating, inviting you into the captivating world of electrochemistry.
Metals in Our Daily Lives
Since the Bronze age (about 3300–1200 BC), we have been enjoying what is called a metal-based civilization. Metals are used everywhere. If you look around, you will probably spot at least one object that contains metals, for instance, a bridge, a bike, or a soda can. Metals such as aluminum (Al), titanium (Ti), and magnesium (Mg) are attractive for manufacturing because they are light, strong, and abundant. These light metals are widely used in many objects, such as cars, planes, computers, phones, and even in medical implants and batteries.
Where do Light Metals Come From?
Like most of the metals we use, light metals must be extracted from a rock (or ore) because they do not exist as pure metal in the Earth’s crust. Aluminum, for example, is extracted from an ore called bauxite (Figure 1), which consists mainly of aluminum oxide [1]. An oxide is a metal that is combined with oxygen. To transform an oxide into a metal, such as making bauxite into metallic aluminum, all the oxygen must be removed from the rock. This requires a lot of energy.
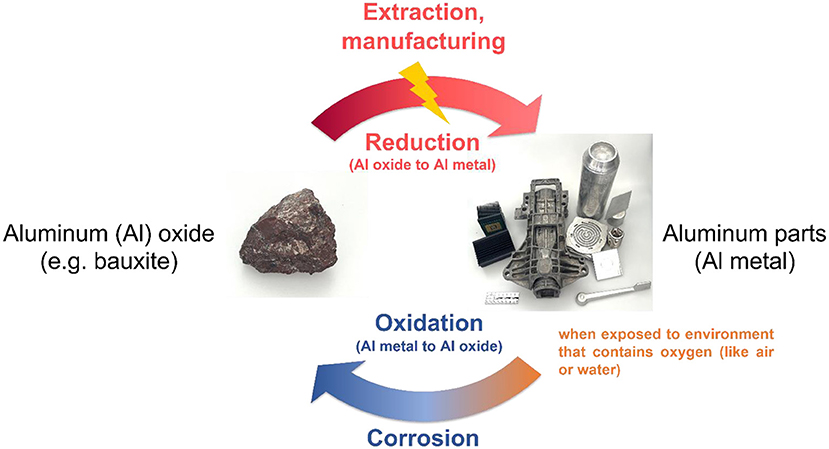
- Figure 1 - The lifecycle of aluminum as an example metal.
- In nature, aluminum (Al) exists as a rock called bauxite, which contains Al combined with oxygen, as Al oxide. Through industrial processes, the metal can be extracted and manufactured into the aluminum objects we use in our daily lives. These processes require a lot of energy. When metallic aluminum reacts with oxygen in the environment (in air or water), it can turn back into aluminum oxide through the process of corrosion (commonly seen as rust on metal objects).
The environment around us—the air we breathe and the water we drink—contains oxygen. Metals can react with environmental oxygen and turn back into oxides (Figure 1) [2]. Rust is the oxide that iron or steel form when they react with their environment. The scientific name for rusting is Corrosion. Corrosion can eventually lead to the metallic object breaking.
Protecting Metals from Corrosion
To prevent corrosion from happening, metals must be protected from their environment. It is a bit like the way we protect ourselves from our environment by putting on a jacket when it is cold outside or wearing sunscreen or sunglasses on a sunny day. The same principle can be applied to metals. In certain environments, some metals can protect themselves by forming a thin, highly stable layer of oxides known as a barrier layer. Aluminum immediately creates this barrier layer, when it is in contact with air or tap water. As a can, aluminum can meet acidic environments such as tomato sauce or soft drinks, and its barrier layer can no longer protect it. So, we need to protect it with a different protective coating. The coating can be paint, a layer of another metal, or a layer of oxide that we grow in a controlled way.
What is Anodizing?
Most of the time, corrosion needs to be prevented because it is uncontrolled. But if we can control it, we can use it for good! By corroding the surface of light metals in a controlled way, we can create a robust, protective coating that shields the metal from the environment. This process is called anodization, a word that comes from “anode”, the metal that oxidizes (Figure 2) [3, 4].
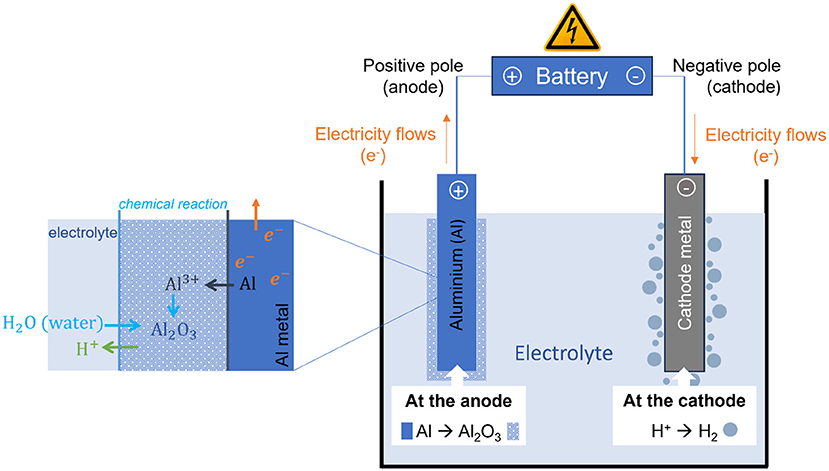
- Figure 2 - To anodize aluminum, the aluminum (anode) is placed in a special liquid (the electrolyte) and connected to the positive side of a battery.
- The negative side of the battery is connected to another metal that does not react (cathode). When both metals are in the electrolyte, the electrical circuit is closed: the battery pushes electrons (electricity) toward the cathode, while ions (tiny, charged particles) move through the liquid between the anode and cathode. Aluminum, as the anode, corrodes (Al metal → Al+3 ions) and can therefore react with oxygen (O2- ions), present in the electrolyte, to form an oxide layer (Al2O3). At the cathode, the electricity flows in, and hydrogen is produced. Protons (H+ ions) in the electrolyte, responsible for its acidity, form hydrogen bubbles (H2).
The anodization process is quite simple. We just need a battery, a container, and an appropriate liquid, which can be as simple as Coca-Cola or lemon juice. Anodizing is an electrochemical reaction, which is a chemical change (transforming the metal into an oxide) that happens when an electric current is applied. Specifically, anodizing consists of passing an electric current between the metal to be protected and another metal that does not react (called an inert metal). To do this, the metal to be protected (aluminum, for example) is connected to the positive pole of a battery. This is the anode, the metal that oxidizes. On the other side, the inert metal is connected to the negative pole. This is the cathode. Think of the cathode as where the electricity goes. To close the electrical circuit between the anode and the cathode, we need a special liquid that conducts the electricity (for instance, Coca Cola). This liquid is called the electrolyte. On the anode, the metal oxidizes—meaning when it encounters oxygen, it combines with it and forms a layer of oxides that grows over time, creating the protective layer (Figure 2). Because the metal at the cathode does not react, the flow of electricity produces hydrogen (this reaction is called hydrogen reduction). In an electrochemical system, oxidation happens at the anode and reduction happens at the cathode, and these two reactions always coexist.
Anodizing Titanium, The Rainbow Metal
Another nice thing about anodizing is that we can decide what kind of layer we want. Depending on the amount of electricity that we apply (voltage) and the kind of electrolyte we use, anodizing can produce a very thin barrier layer or a rather thick, porous layer on the metal (Figure 3). Barrier layers form in slightly acidic electrolytes, such as citric acid (you might know this as lemon juice). They are only a few hundred nanometers thick, which is 200 to 1,000 times thinner than a human hair. These barrier layers are still strong enough to protect the aluminum alloys used in aircraft. But the protective layers can also look nice! For instance, barrier layers formed on titanium can take almost all the colors of the rainbow. Therefore, titanium is sometimes called “the rainbow metal”. Oxidized titanium is often used to decorate jewelry or objects like keys, phones, or computer cases. The colors are also used by dentists to distinguish the size of implants intended to replace damaged teeth. The color of the barrier layer formed on titanium depends on the thickness of the layer. In general, the more electricity we provide, the thicker the barrier layer. Almost every color can be obtained by varying the amount of electricity applied (Figure 3A).
The Porous Layer can be a Fascinating Container
What if we want our protective layer to protect against corrosion, but also against wear or heat? We can do this by creating a porous layer. If we use aluminum, a battery, and Coca-Cola, we can create a porous oxide layer much thicker than a barrier coating. The thickness of porous layers can reach over 200 µm (three times the thickness of a human hair).
When you bake muffins, you put the dough in the muffin-baking molds and (usually) end up with delicious pastries. Porous layers work in a similar way. Instead of dough, we can add substances inside the pores—the tiny holes in the coating to give the coating an additional function or use. A functional coating is a coating that can do things the coating would not otherwise be able to do. For example, by adding dyes to the pores, we can color the typically gray porous layers formed on aluminum, giving them any color we want (Figure 3B). Since we can modify porous coatings, they can have many uses. These colors can be used to protect aluminum building exteriors and give them a natural look. We can also use the pores to hold antibiotics to prevent infections in our bodies, or to house other substances such those that reduce wear on moving parts like gears or prevent corrosion. They can even be used as molds to manufacture nano-objects.
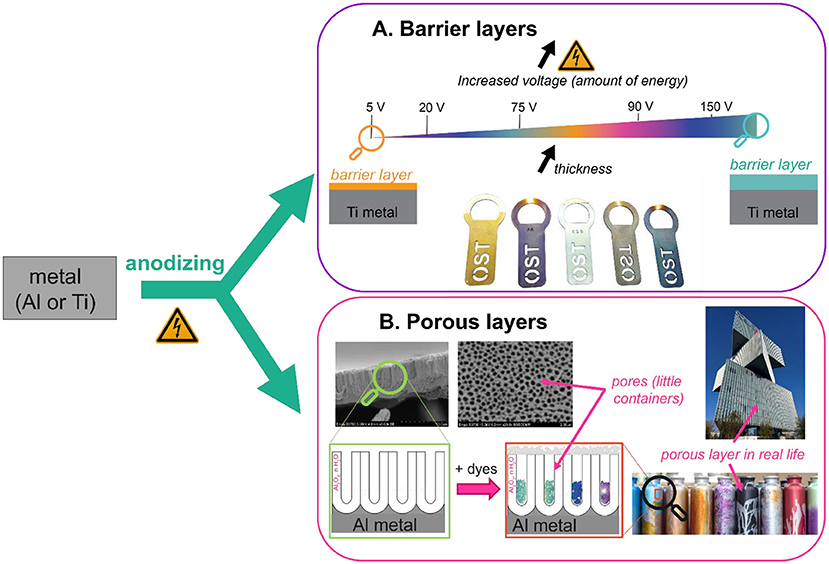
- Figure 3 - Anodizing can create barrier or porous layers.
- (A) To create barrier layers, titanium (Ti) bottle openers were anodized in Coca Cola. By varying the voltage, different TiO2 thicknesses are obtained (all at least 200 times thinner than a human hair), creating different colors. (B) Aluminum (Al) water bottles and the façade of the Nhow Hotel in Amsterdam were anodized to form a thicker, porous layer (up to three times the thickness of a human hair). Dyes were added inside the pores to give the objects a nice appearance (Source: BWB Surface Treatments).
Can We Anodize All Metals?
No, only some metals can be anodized. They are called “valve metals”, like aluminum, titanium, tantalum, and niobium. They can be anodized because they only allow the electrical current (or electricity) to pass in one direction.
Why is Anodizing so Important?
Now you know that corrosion has a “good” side—it can be used to produce coatings with special properties. We can shield light metals and prevent them from corroding very fast in environments such as seawater or the atmosphere by covering them with a protective layer through an electrochemical reaction that leads to oxidation. A process called anodization. This inexpensive and simple method can be modified in lots of ways to create all sorts of colored surfaces and even new materials. Anodizing is an incredible solution to protect light metals and increase the life of many important objects and devices in our daily lives—cars, planes, computers, phones, and more.
Glossary
Oxide: ↑ A metal combined with oxygen. For instance, alumina, Al2O3, is one type of Al oxide.
Corrosion: ↑ Corrosion is the gradual degradation of a metal object by reaction with its environment. When iron or steel corrode, they form an orange product known as rust.
Anodization: ↑ Electrochemical process that, by oxidizing a metal, forms a stable and protective oxide layer on a metal.
Electrochemical Reaction: ↑ An electrochemical reaction is when electricity and chemicals work together to make changes in a material.
Anode: ↑ The side of an electrochemical system where a chemical reaction happens that gives off electrons (oxidation). The electricity goes away from the anode.
Cathode: ↑ The side of an electrochemical system where a chemical reaction happens that receives electrons (reduction). The electricity comes into the cathode.
Electrolyte: ↑ A special liquid or paste that conducts electricity using the movement of tiny, charged particles called ions. For anodizing, it can be as simple as Coca Cola or lemon juice.
Porous: ↑ A material full of tiny openings, like a sponge. In anodizing, a porous layer is a coating with tiny holes that can be filled with dyes or other substances.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
AI Tool Statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
References
[1] ↑ Polmear, I., StJohn, D., Nie, J.-F., and Qian, M. (eds.). 2017. Light Alloys., 5th Edn. Boston, MA: Butterworth-Heinemann.
[2] ↑ Landolt, D. 2007. Corrosion and Surface Chemistry of Metals., 1st Edn. Lausanne: EPFL Press. doi: 10.1201/9781439807880
[3] ↑ Runge, J. M. 2018. The Metallurgy of Anodizing Aluminum., 1st Edn. Cham: Springer. doi: 10.1007/978-3-319-72177-4
[4] ↑ Wandelt, K. (ed.). 2018. Encyclopedia of Interfacial Chemistry – Surface Science and Electrochemistry. Amsterdam: Elsevier.
