Abstract
Ore deposits are masses of ore (rocks rich in metal) that are mined to obtain the metals needed for the machines and devices we use in everyday life, but how do ore deposits form, and where do we find them? Nowadays, recycling supplies some metals but by no means all of them, and not in sufficient amounts. So, for many years to come, we will continue to depend on ore deposits. To improve the chances of finding new deposits, geologists need to understand what processes concentrate metals into ores. This is the goal of scientific research on ore formation, and the best method is to drill boreholes through a deposit to obtain a continuous series of rock samples—drill cores—from top to bottom. The Bushveld drilling project in South Africa is described here as an example. This project targets the world’s largest ore deposit of platinum, a key metal for green energy technologies.
Introduction
This article describes what ore deposits are and how they form. There are still many open questions, and this is a hot research topic because all the metals we depend on for everyday life come from ore deposits. The demand for metals keeps increasing as the world’s population grows and we shift from fossil fuels to environmentally friendly green energy. Recycling helps supply some metals but not enough to satisfy the demand [1]. Figure 1 shows supply and demand curves for platinum, a high-value metal in great demand for green energy technologies. These are just predictions but according to the plot, the supply of platinum from mining and recycling will not meet the rising demand. This means we must either increase the supply—by finding new deposits or through better recycling—or reduce the demand.
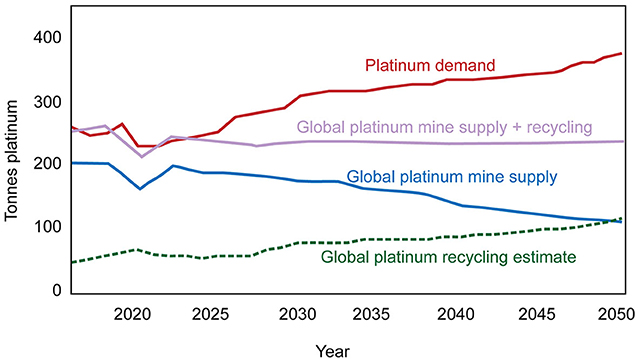
- Figure 1 - Estimated supply and demand curves for platinum.
- Platinum supplies from mining (blue) and recycling (green) are combined to create the violet curve. According to these curves, platinum demand (red) will exceed supply soon, unless mining or recycling are increased or the demand for platinum is reduced [Figure adapted from this website and used with permission; platinum demand data from [3]].
What Are Ore Deposits?
Ore deposits are places on the Earth where geologic processes have concentrated metals enough that we can mine them efficiently. The metals in most ore deposits were originally dissolved and transported in fluid deep in the earth, often over long distances, before crystallizing in the form of solid ore. Also, very few metals exist in their pure form in nature. Gold is an exception, but almost all others combine with elements like oxygen, carbon, or sulfur to form what we call ore minerals. After mining, ore minerals must be processed to obtain the pure metal. Processing uses lots of energy and water, and the leftover materials must be recycled or stored in waste dumps. These are negative aspects of mining.
There many kinds of ore deposits [1], so this article describes just the two that are the most important source of metals for green energy technologies. They are called hydrothermal and magmatic deposits, and the difference between them is the way metals are dissolved and transported. In hydrothermal deposits, metals are dissolved in hot water (think of hot springs), but in magmatic deposits, metals are carried in liquid molten rock called magma (think of lava).
Hydrothermal deposits are often found in quartz veins that fill open cracks in rocks (Figure 2). Hydrothermal ores were transported by hot fluids circulating in the Earth, so it is easy to see why they are found in cracks and fissures like this vein. But hot fluids can also permeate (soak through) porous rocks like sandstone or limestone. This process forms hydrothermal ores that are spread out through the porous rocks.

- Figure 2 - Co-author Marta Codeço in front of a hydrothermal quartz vein (wide white stripe through the rock) in the Panasqueira mine, Portugal.
- This mine is the second-largest source of tungsten in Europe (Photo credit: Robert Trumbull).
Magmatic deposits contain metals that were transported by molten rock called magma. Most people know magma as lava flowing from a volcano, but it forms deep in the Earth. The melting temperature of rocks is around 1,000°C, but the exact temperature depends on the minerals that the rock contains. If melting minerals contain metals, they are released into the magma. Since magma is liquid and usually less dense than solid rock, it flows upwards through the crust. Sometimes magma erupts at the surface as lava or volcanic ash. But deep beneath every volcano, there is a reservoir of magma called a magma chamber. Ore deposits can form in both the surface environment and in magma chambers, as explained below.
How Do Hydrothermal Ore Deposits Form?
Hydrothermal deposits form by the interaction of hot water, called hydrothermal fluid, with rocks. Hydrothermal fluids are between about 200 and 500°C, and they react chemically with the rocks they pass through, causing minerals in the rocks to dissolve and releasing metals into the fluid. Exactly which metals and how much of them dissolve depends on the solubility of the minerals that contain them. For example, rock salt is a mineral that is very soluble in water, even at room temperature. Other minerals like feldspars and micas are much less soluble than salt, but they can dissolve at high temperatures, releasing metals like lead, iron, titanium, tin, and lithium into the hydrothermal fluid.
Once they are dissolved, metals can move for long distances through cracks and pores. Ore deposits form when the metals come out of the solution again and crystallize as ore minerals. Crystallization often happens when the hydrothermal fluid flows into colder rocks and cools—think of rock candy growing from a sugar solution. Other processes like boiling, reaction with oxygen, or changes in pH can also cause crystallization. Geologists study ore deposits in detail to find out what caused the ore minerals to crystallize where they did. This knowledge helps them decide where to look for more.
How Do Magmatic Ore Deposits Form?
Magmatic ore deposits form by melting minerals in deep rocks, transporting the metals upwards in the liquid magma, and crystallizing the ore minerals to form a deposit. There are two places where this crystallization happens. One is near or at the surface of volcanoes. Because the pressure is very low, bubbles form in the magma—think of opening a bottle of a fizzy drink. This process is called degassing (loss of gas), and it releases water vapor, carbon dioxide, and sulfur into the air. Degassing is why some volcanoes have a plume of “smoke” (really steam) above them, and why they stink of sulfur. Degassing can form ore deposits because the hot fluids released contain metals. Technically, these are hydrothermal deposits because they form from hot fluids. However, because the fluids come from magma, we use a special term: magmatic-hydrothermal. Much of the world’s copper comes from magmatic-hydrothermal deposits. The crystallization of ore minerals from magmatic-hydrothermal fluids follows the same processes just described for hydrothermal deposits.
The second place where magmatic ores form is in magma chambers. These are located deep enough (several km down) that magma cools slowly—over hundreds or thousands of years. This gives time for ore minerals to crystallize and separate from the liquid magma by floating or sinking within the chamber. If the crystals form layers, the metal content in the layers can be high enough for mining (Figure 3). Thus, ore formation in magma chambers is all about crystallizing ore minerals and concentrating them into mineable deposits. Many factors play a role here. The temperature and composition of magma determine which minerals crystallize and when. The density determines if crystals sink or float. The magma fluidity affects how fast the crystals separate. And the cooling rate determines if there is enough time for crystals to settle into layers before the magma solidifies. Geologists must understand these factors to find new deposits.

- Figure 3 - Rock outcrops containing magmatic chromium ore (black layers) in the Bushveld Complex, South Africa.
- The ore layers contain about 50% by weight of chromium metal. They formed by crystal accumulation in a magma chamber (Photo credit: Wikimedia Commons, CC-BY-2.0).
Why Is Scientific Drilling Important?
It is uncommon to find ore deposits exposed on Earth’s surface. Usually, they are at least partly covered by grass, trees, snow, or desert sand, or they may be deeply eroded. So, to study ore deposits in detail, there is nothing better than drilling into the Earth and pulling up a cylinder of rock called a drill core. Drill cores are thin (typically 5–10 cm in diameter), but they can be hundreds or even a few thousand meters long. Furthermore, many drilling projects make more than one hole, so they can get a 3D record of the rocks beneath our feet.
A detailed 3D record of ore deposits lets geologists search for features in the ores and the surrounding rocks that give clues to the ore-forming process. Examples are cracks and porous zones in the rock, changes in color or texture, and changes in the type and abundance of minerals. The next step is to take samples to the laboratory and determine the metal content and the kinds of ore minerals present. Drill cores are ideal for chemical and mineral analyses because they are “fresh from the ground,” unaffected by years of weathering and erosion at Earth’s surface.
The International Continental Scientific Drilling Program (ICDP) is a multi-national organization that provides money and technical advice to research projects that use drilling for scientific research. The largest ICDP project to study ore deposits is the Bushveld Drilling Project (BVDP) in South Africa. The target of the BVDP is the world’s largest magmatic ore deposit of platinum and related metals [2]. These metals have many applications, and a surge in demand is expected for green-energy technologies [3]. The Bushveld platinum ores are found as layers in an ancient, now rock-solid magma chamber (Figure 3). No one knows exactly how these layers formed, and the only way to find out is to study them in detail, together with the rocks above and below. When completed in 2024, the BVDP project will have collected more than 10 kilometers of drill cores through the magma chamber and its ore layers. This collection of drill cores will be studied by international teams of geologists to work out how the ore layers formed. If we can answer that, it will be easier to find other platinum deposits in the future.
Looking Ahead
Platinum is one of several metals that are essential ingredients for environmentally-safe sources of energy, so our future depends on an adequate supply. More and better recycling is going to be part of the solution, but ore deposits will be the main source of metals for many years to come. As today’s deposits are depleted by mining, new ones must be found. Scientific research plays a key role here, because the Earth is a big place and knowing where to find new ore deposits requires understanding how they form.
Glossary
Green Energy: ↑ Green energy describes energy that comes from renewable sources like wind or solar power.
Magmatic Deposit: ↑ An ore deposit formed by crystallization from a magma.
Hydrothermal Deposit: ↑ An ore deposit formed by crystallization from a hydrothermal fluid.
Porous: ↑ A material is said to be porous when it contains open spaces or pores, like a sponge.
Hydrothermal Fluid: ↑ The term literally means hot aqueous fluid, generally water rich in dissolved salt and minerals at temperatures from 200 to 500°C.
Solubility: ↑ This term describes how much of a substance can be dissolved in another.
Crystallization: ↑ The process of forming crystals by transformation from liquid to solid or by exceeding the limit of solubility.
Degassing: ↑ The process of losing gas from a solution by exceeding the limit of solubility, like CO2 released from carbonated water.
Drill Core: ↑ A cylinder of rock extracted from the ground by drilling. Drill cores are usually 5–10 cm thick and from several hundred meters to over 3,000 m long.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
MC was supported by the DFG Deutsche Forschungsgemeinschaft Priority Program 2238 “Dynamics of Ore Metal Enrichment (DOME)” grant GL945/1-1.
References
[1] ↑ Arndt, N. T., Fontboté, L., Hedenquist, J. W., Kelser, S. E., Thompson, J. F., and Wood, D. G. 2017. Global mineral resources. Geochem. Perspect. Eur. Assoc. Geochem. 6:1–171. doi: 10.7185/geochempers.6.1
[2] ↑ Mungall, J. E., and Naldrett, A. J. 2008. Ore deposits of the platinum-group elements. Elements 4:253–8. doi: 10.2113/gselements.4.4.253
[3] ↑ Smith, B. J., Graziano, D. J., Riddle, M. E., Liu, D.-J., Sun, P., Iloeje, C., et al. 2022. Platinum Group Metal Catalysts: Supply Chain Deep Dive Assessment. Washington, DC: United States Department of Energy. doi: 10.2172/1871583
