Abstract
Ammonia is a chemical commonly found in fertilizers, cleaning products, and even animal waste. While this substance does have some important uses, rising emissions of ammonia in the atmosphere can pose serious risks to human health, plant and animal health, and the environment. Therefore, measuring and tracking ammonia levels in the air is crucial, especially as human activities, such as large-scale farming and industrial processes, release more and more ammonia. In this article, we will tell you about the main sources of ammonia pollution, the dangers it poses to health and the environment, and how scientists are using satellites to monitor ammonia levels from space. By keeping a close eye on ammonia trends, researchers hope to trigger ways to reduce emissions and protect the planet for future generations.
Ammonia is no Joke!
What do some cleaning products, garden fertilizer, and chicken poop have in common? While this might sound like the start of a corny “dad joke”, there is no punchline—but there is a scientific answer! All three of these substances contain a chemical called ammonia. Maybe you have noticed the sharp, strong smell of ammonia in a recently cleaned school bathroom or gym locker room, or while emptying a cat’s litterbox. Even if you have heard of (or smelled!) ammonia before, you might not be aware that scientists are actively monitoring ammonia levels in the air we breathe, both from land and from space. This is important because too much ammonia can be harmful to humans, animals, plants, and the environment. In the rest of this article, we will tell you about the sources of ammonia, why scientists are concerned about ammonia levels in the atmosphere, and how they monitor it from space.
Where Does Ammonia Pollution Come From?
In its pure form, ammonia is a gas with the chemical formula NH3, meaning that each molecule has a nitrogen atom surrounded by three hydrogen atoms (Figure 1A). Ammonia is produced by both natural sources and anthropogenic (human-caused) processes (Figure 1B).
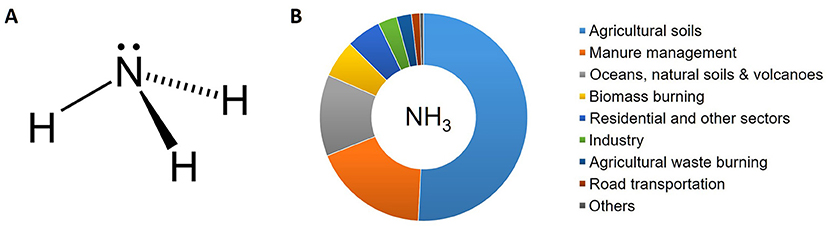
- Figure 1 - (A) Ammonia is made up of a nitrogen atom (N) surrounded by three atoms of hydrogen (H), forming a molecule with a pyramidal shape.
- (B) Main contributors to ammonia emissions in the air. You can see that the vast majority of ammonia emissions are from anthropogenic activities like farming and industry.
When dead plants and animals decompose (break down) in nature, ammonia is released into the soil and air. Living animals, including humans and livestock like cows, chickens, and pigs, contribute to ammonia production too, when their waste products (pee and poop) are decomposed by bacteria. Oceans also emit ammonia, and wildfires can generate large amounts of this gas as they burn trees and other plants.
Human activities, however, produce much more ammonia than natural processes. The giant farms that grow our food are by far the biggest anthropogenic source. Man-made fertilizers contain ammonia because it provides a readily available source of nitrogen, which plants need to grow. When farmers use large amounts of fertilizers on their crops, some of the ammonia gets released into the air. Huge cow, chicken, and pig farms also generate large amounts of ammonia from animal waste, as we mentioned above. Overall, in Europe, the United States, and China, livestock waste and fertilizer use account for over 80% of total ammonia emissions.
Besides farming, other anthropogenic sources of ammonia include industries that produce fertilizers, steel, explosives, and other chemicals. Waste treatment plants and gas-powered vehicles also contribute to ammonia pollution, though not nearly as much as agricultural sources.
Why Should We Worry About Ammonia?
So, what makes ammonia so bad? At high levels, there are three main worries: human health problems, biodiversity loss, and climate change.
Human Health Problems
When ammonia mixes with other pollutants in the air, it can form tiny particles called particulate matter. These particles can be easily inhaled into the lungs due to their small size, leading to a variety of breathing issues like asthma, bronchitis, and other long-term respiratory illnesses. Particulate matter can affect more than just the lungs—it can also enter the bloodstream and impact other parts of the body [1, 2]. This can increase the risk of heart disease, stroke, and other serious health issues that can shorten people’s lives. Children and the elderly are often the most vulnerable to health problems caused by particulate matter pollution.
Biodiversity Loss
Ammonia released into the atmosphere can also settle back to Earth, where it can change the chemistry of the soil and water. These changes can harm and even kill plants and animals by disrupting the natural balance in ecosystems, leading to a loss of critical biodiversity. For example, too much ammonia can lead to nutrient overload in water bodies, causing excessive growth of tiny plants called algae. This process is called eutrophication. As they grow, the algae use up all the oxygen in the water, creating “dead zones” where fish and other aquatic life cannot survive. By altering the chemistry of soil, ammonia can also affect the types of plants that can grow and thrive, further decreasing biodiversity.
Climate Change
Ammonia plays a relatively small but interesting role in climate change, too. It has both cooling and warming effects on the climate. One cooling effect relates to the formation of particulate matter, mentioned above. These tiny particles are efficient at reflecting sunlight back into space and thereby cool down our planet. Ammonia also influences the way plants grow, which in turn affects how much carbon dioxide (the main greenhouse gas responsible for climate change) is absorbed by plants and removed from the atmosphere. There are many other complex effects of ammonia on the climate. After considering the most important ones, most scientists around the world agree that ammonia cools down the climate a little, but not nearly enough to stop global warming.
So, now you know why keeping ammonia levels in check is important—but how do scientists monitor ammonia levels to understand whether they are changing over time?
Keeping Track of Ammonia With Satellites
One way to measure ammonia in the air we breathe is by using instruments that can directly sample the air to check ammonia levels. Ammonia-detecting instruments can be found in air-sampling stations in various countries, and they can provide very accurate data. However, ground-based instruments can only measure ammonia in the specific places where they are located. There are not enough ground-based instruments spread out all over the world to give scientists the “big picture” of ammonia emissions.
To overcome this limitation, scientists can use satellites to monitor ammonia levels from space. Satellites can carry advanced instruments that can detect ammonia and other gases in the atmosphere by measuring the specific colors of light that these gases absorb and emit. Because they are “looking down” at Earth, satellites can show scientists how ammonia is distributed over vast areas, including remote places that cannot be measured with ground-based instruments.
For our study [3], we used 11 years of data (2008–2018) from a satellite-based instrument called the Infrared Atmospheric Sounding Interferometer (IASI). IASI takes measurements all over the Earth twice a day, and it is very sensitive, meaning it can accurately detect much smaller amounts of ammonia than previous satellite-based instruments could. We used data from IASI to understand where atmospheric ammonia is coming from, how it spreads, and how levels are changing over time.
Ammonia is Increasing in Earth’s Atmosphere
Data from IASI allowed us to pinpoint hundreds of ammonia sources, including major “hot spots” where a lot of ammonia is being released in a small area [4, 5]. Most of the hot spots were areas where intensive livestock farming and industrial activities were happening (Figure 2). We also created detailed maps of the Earth, showing how ammonia emissions changed for countries and regions over the 11-year measurement period.
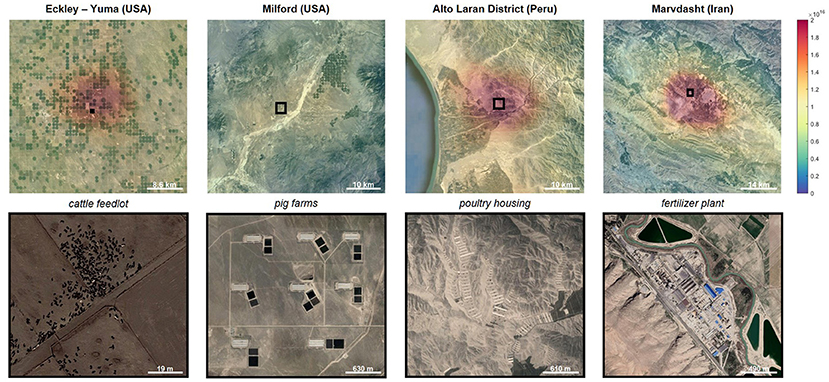
- Figure 2 - Examples of ammonia mega-emitters seen from space.
- (Top row) Atmospheric ammonia concentrations measured using IASI, overlaid on images of the ground. The color scale shows increasing ammonia concentrations, with blue representing low concentrations and red representing high concentrations. (Bottom row) Close-up view of the areas outlined in black in the top panels, showing the ammonia emitter (Image modified from Clarisse et al. [4] and Van Damme et al. [5] with permission).
Looking at the whole world, we saw an increase in ammonia levels by about 12.8% from 2008–2018. But this increase was not consistent all over the Earth—some areas saw big increases while others experienced decreases (Figure 3) [3]. East Asia, for instance, showed the largest increase in ammonia levels, with an increase of >75% over the observation period. Even in places like the European Union that have policies in place to try to reduce ammonia emissions, we still saw increases during the observation period.
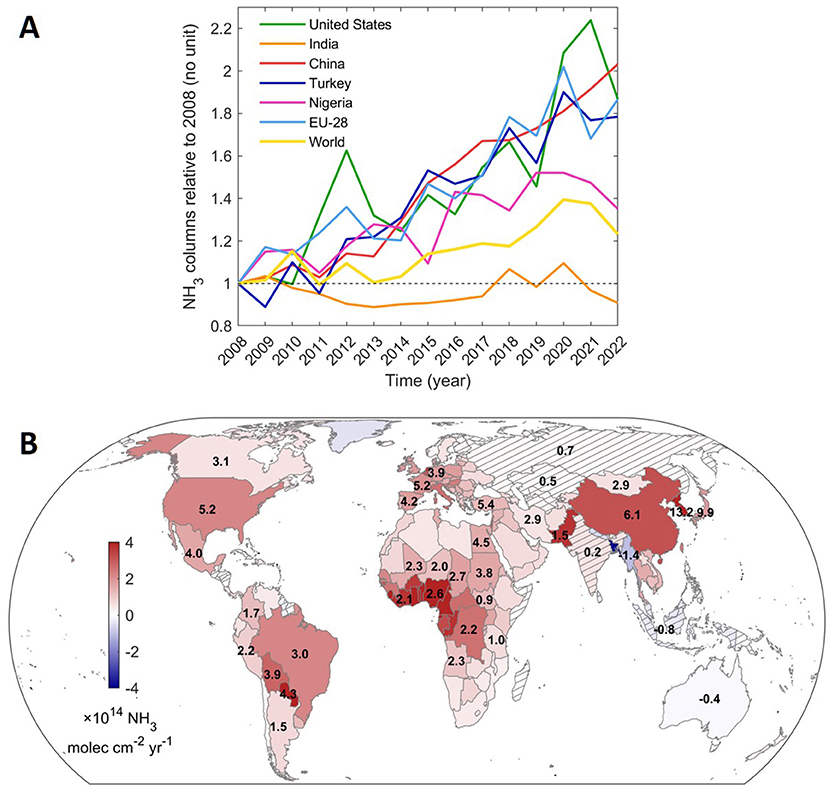
- Figure 3 - (A) Changes in the ammonia concentration from 2008–2022 (relative to 2008 levels) as monitored by IASI.
- Trends are shown for selected countries, Europe (EU-28), and the entire world. (B) Trends for each country over the same period. Red means increase and blue means decrease of NH3 in the air. Relative trend values (in %) are included in black for selected countries (Image modified from Van Damme et al. [3] with permission).
Why is Ammonia Rising?
A combination of two factors explains why ammonia concentrations in the air are rising: increasing ammonia emissions and changes in air pollution control measures that cause ammonia to last longer in the atmosphere.
The most obvious reason for rising ammonia levels is simply because more ammonia is being released into the air, primarily through increases in farming and industrial activities. In East Asia, for example, both factories and large-scale farming increased during the observation period. Fires—both wildfires and fires used to clear land for farming—also contribute to increased ammonia in the atmosphere. For example, in Indonesia, extensive peatland fires in 2015 released significant amounts of ammonia, and in Nigeria, “slash-and-burn” forest clearing for agriculture contributed to rising ammonia levels during the observation period.
The second reason, air pollution control, might not seem to make sense at first. If ammonia is a type of air pollutant, why would controlling air pollution make atmospheric ammonia levels worse? The answer lies in the interaction between ammonia and other air pollutants. Remember how we said that ammonia can react with other pollutants to form particulate matter? The formation of those tiny particles reduces ammonia levels in the air. However, many countries have air pollution laws in place to reduce the pollutants that pull ammonia out of the air. When levels of those pollutants are reduced, ammonia stays in the atmosphere longer, leading to higher overall levels.
Why is This Work Important?
As we have explained, understanding and addressing rising ammonia levels is crucial for protecting both human health and the environment. Data from IASI show that current air pollution control policies are not enough to keep ammonia emissions in check. Even in regions with fairly strict air pollution laws, ammonia levels are still rising. This tells us that new, more effective strategies are needed to mitigate (reduce) ammonia emissions.
Mitigation could include better farming practices, such as using more efficient fertilizers and improving animal waste management. Industries could also adopt cleaner technologies and processes to reduce the amount of ammonia they release. For example, advanced filtration systems could be installed to help capture ammonia, keeping it out of the air.
It is important to keep an eye on how well pollution-control efforts are working. Satellite data, like that from IASI, help scientists monitor ammonia levels all over the world. By watching these levels closely, scientists can tell if efforts to reduce ammonia are actually making a difference or if we need to make changes to our plans. Satellite tracking is one way to ensure that ammonia mitigation efforts are successful, helping countries do their best to keep humans, the environment, and the planet safe from the dangers of air pollution and ecosystems degradation.
Glossary
Ammonia: ↑ A gas made of nitrogen and hydrogen (NH3), found in fertilizers, cleaning products, and animal waste. In excess, it can be harmful to human health, the environment, and air quality.
Anthropogenic: ↑ Caused by human activities, such as farming, industry, and pollution. Anthropogenic processes often contribute to environmental issues like climate change, deforestation, and air or water pollution.
Biodiversity: ↑ The variety of life in a particular habitat or ecosystem, including all plants, animals, and microorganisms. High biodiversity helps ecosystems stay healthy and resilient to changes or threats.
Particulate Matter: ↑ Tiny particles in the air, like dust, dirt, soot, or chemicals, that can be inhaled into the lungs. Particulate matter can cause health problems, especially for the respiratory and cardiovascular systems.
Eutrophication: ↑ A process in which excess nutrients, like ammonia, cause rapid algae growth in water bodies. This depletes oxygen in water, creating “dead zones” where aquatic life cannot survive.
Climate Change: ↑ A long-term change in Earth’s climate, especially a rise in average global temperatures, primarily caused by human activities like burning fossil fuels, deforestation, and industrial processes.
Mitigation: ↑ Actions taken to reduce or prevent the harmful effects of something, such as limiting emissions or improving practices to lessen environmental damage or health risks.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This study was co-written by Susan Debad Ph.D., graduate of the UMass Chan Medical School Morningside Graduate School of Biomedical Sciences (USA) and science communicator at SJD Consulting, LLC. This study was supported by the BELSPO and ESA-Prodex arrangements (IASI.Flow and HIRS), F.R.S.-FNRS, ULB Foundation, Air Liquide Foundation (TAPIR project).
Original Source Article
↑Van Damme, M., Clarisse, L., Franco, B., Sutton, M. A., Erisman, J. W., Kruit, R. W., et al. 2021. Global, regional and national trends of atmospheric ammonia derived from a decadal (2008–2018) satellite record. Environ. Res. Lett. 16:055017. doi: 10.1088/1748-9326/abd5e0
References
[1] ↑ Plautz, J. 2018. Ammonia, a poorly understood smog ingredient, could be key to limiting deadly pollution. Sci. News. doi: 10.1126/science.aav3862
[2] ↑ Thangavel, P., Park, D., and Lee, Y. C. 2022. Recent insights into particulate matter (PM2.5)-mediated toxicity in humans: an overview. Int. J. Environ. Res. Public Health 19:7511. doi: 10.3390/ijerph19127511
[3] ↑ Van Damme, M., Clarisse, L., Franco, B., Sutton, M.A., Erisman, J.W., Wichink Kruit, R., et al. 2021. Global, regional and national trends of atmospheric ammonia derived from a decadal (2008–2018) satellite record. Environ. Res. Lett. 16:055017. doi: 10.1088/1748-9326/abd5e0
[4] ↑ Clarisse, L., Van Damme, M., Clerbaux, C., and Coheur, P.-F. 2019. Tracking down global NH3 point sources with wind-adjusted superresolution. Atmos. Meas. Tech. 12, 5457–5473. doi: 10.5194/amt-12-5457-2019
[5] ↑ Van Damme, M., Clarisse, L., Whitburn, S., Hadji-Lazaro, J., Hurtmans, D., Clerbaux, C., et al. 2018. Industrial and agricultural ammonia point sources exposed. Nature 564, 99–103. doi: 10.1038/s41586-018-0747-1