Abstract
Have you ever thought about what helps you do things like walking, turning pages, getting dressed, or standing up from a chair? It is a special network in your brain that sends signals to your muscles to help you move. When this network gets damaged, it can cause problems like shaking limbs, stiff muscles, and moving slowly. This happens to people with Parkinson’s disease, making simple tasks like turning pages or drinking a cup of tea hard. One cool treatment for this disease is called deep brain stimulation (DBS). DBS sends electric pulses to the brain to fix the signals, restoring the proper connection between the brain and muscles. As a result, this treatment helps people with Parkinson’s disease move better and do their everyday activities more easily.
What is Parkinson’s Disease?
Parkinson’s disease (PD) is a condition that affects the brain. In this disease, certain brain cells, called dopaminergic neurons, start to die. These neurons make dopamine, a chemical that helps send signals in the brain and makes our movements smooth and controlled. These important neurons are found in a part of the brain called the substantia nigra, as illustrated in Figure 1 [1]. This area looks black by the naked eye or under a microscope because of a pigment called melanin inside the neurons. The word “nigra” means “black” in Latin. PD sometimes happens because of genetic problems or contact with harmful chemicals, but most of the time, we do not know why it starts [2].
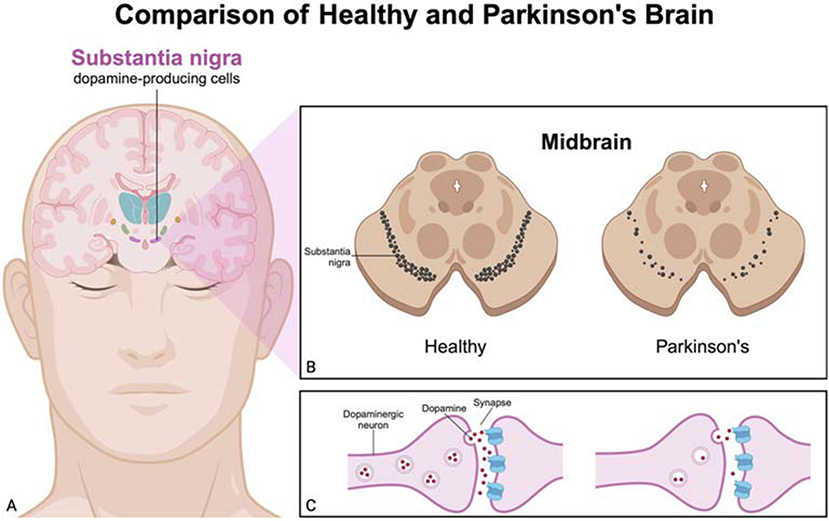
- Figure 1 - Comparison between a healthy brain and a Parkinson’s brain.
- (A) Cross-section of a brain showing the substantia nigra, a dopamine-producing area. (B) Comparison of the substantia nigra in the midbrain between a healthy brain and a brain with PD, highlighting the reduced number of dopaminergic neurons in PD. (C) Dopamine levels in the gaps between two neurons, showing the reduced number of dopamine molecules in PD compared to a healthy brain. This reduction in dopamine leads to the motor symptoms seen in Parkinson’s patients (Created with BioRender.com).
People with PD have specific kinds of motor (movement-related) symptoms, as shown in Figure 2. These include shaking (tremors), stiff muscles (rigidity), slow movement (bradykinesia) or no movement (akinesia), and balance problems (postural instability). PD can also cause non-motor symptoms like mood changes, memory problems, sleep issues, and digestive problems [1]. These symptoms can affect the daily activities of PD patients. Let us explore these symptoms through the story of John.
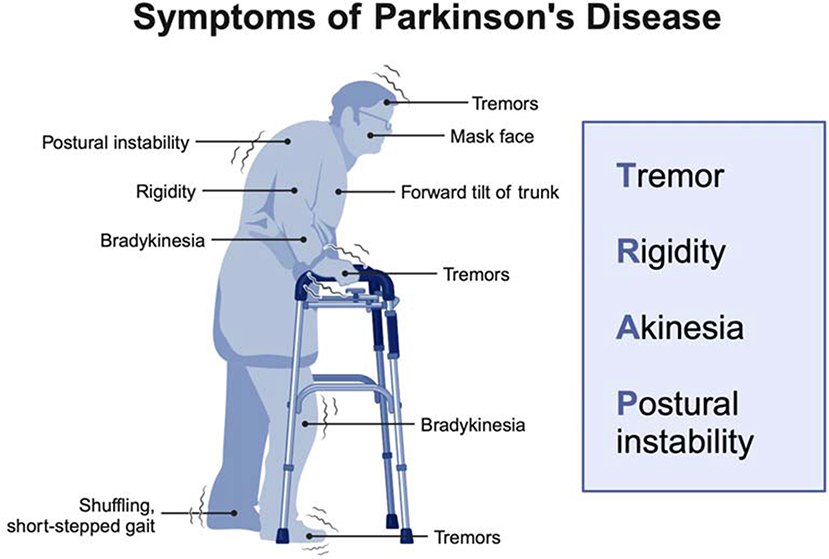
- Figure 2 - Common symptoms in PD patients include tremors (shaking), rigidity (stiff muscles), bradykinesia (slow movement), akinesia (no movement), and postural instability (balance problems).
- Other symptoms include a masked face (reduced facial expressions), a forward tilt of the trunk, and a shuffling, short-stepped gait (Created with BioRender.com).
John, a 65-year-old retired teacher, began noticing symptoms of PD while he was still teaching. He observed tremors when his hand started shaking uncontrollably while he was reading a book. This made it difficult for him to flip the pages or hold a pen. As months passed, he experienced muscle rigidity, causing his body to become stiff. This made his daily commute painful and dressing difficult. Sometimes, he could not move at all, which made it even harder to get out of bed. His movements became slower, turning simple tasks like brushing his teeth into a hard, tiring effort. John’s poor balance and muscle stiffness caused him to have a hunched-over posture and to fall often, both at school and at home. Standing up from a chair or walking without stumbling became daily challenges. Managing these PD symptoms is crucial for his quality of life.
Treatments for Parkinson’s Disease
It is important to note that there is no cure for PD. In the past, doctors treated PD with surgery. Surgeons performed a procedure called thalamotomy, which destroyed a small area of the thalamus (a part of the brain that controls our movements). This surgery helped reduce PD symptoms, especially tremors, by disrupting the abnormal brain activity. Tremors are very noticeable and cause social discomfort. As a result, most surgeries focused on stopping them, even though not being able to move was more disabling. Thalamotomy was the most common surgery until a big shift happened with the arrival of a new medication [2].
The introduction of levodopa changed the way PD was treated— doctors started using medicine instead of surgery. Today, medication remains the main treatment, and levodopa is the most commonly used medication. Levodopa travels through the blood to reach the brain. There, it gets converted to dopamine in the remaining healthy dopaminergic neurons. This helps make up for the lack of dopamine and improves movement in PD patients. Levodopa is effective for about 5 years, and, after that, symptoms worsen. This happens because the dopaminergic neurons continue to die, so the brain produces very little, if any, dopamine. As a result, doctors may need to prescribe higher doses of levodopa over time. However, higher doses can cause unwanted movements [2]. This stage is known as advanced PD, and patients might need additional treatments, such as surgery. Currently, one of the most common surgical treatments for advanced PD is deep brain stimulation (DBS).
Using Deep Brain Stimulation to Treat Parkinson’s Disease
Modern DBS helps relieve symptoms in PD patients. Doctors target three main brain areas for this treatment: the nucleus ventralis intermedius (Vim) of the thalamus, the globus pallidus pars interna (GPi), and the subthalamic nucleus (STN), as shown in Figure 3.
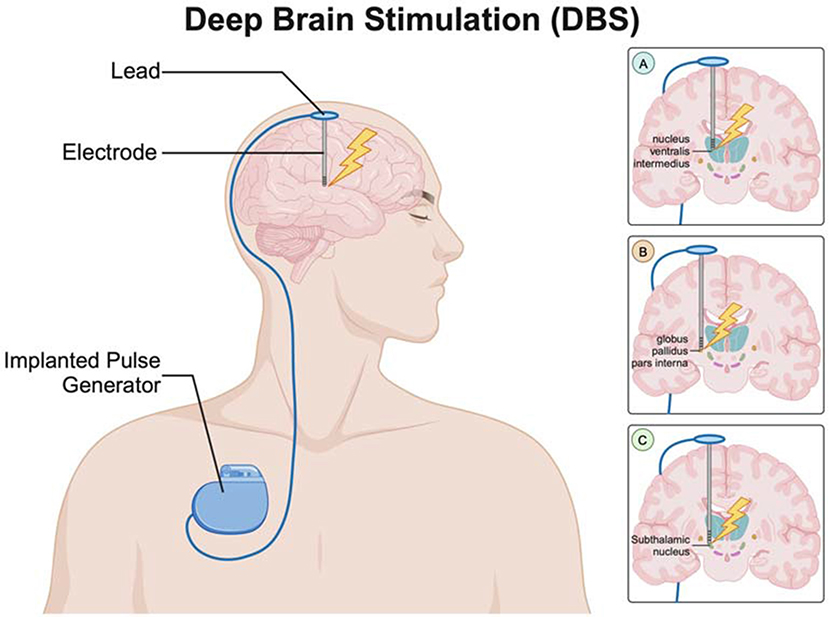
- Figure 3 - The DBS system has three main parts: an implantable pulse generator (IPG), an electrode, and a lead connecting the two.
- The electrodes can be placed in different parts of the brain to help with the specific symptoms each person with Parkinson’s disease has. (A) Electrode targeting the nucleus ventralis intermedius (Vim) of the thalamus. (B) Electrode targeting the globus pallidus pars interna (GPi). (C) Electrode targeting the subthalamic nucleus (STN). Each of these targets helps to manage different symptoms of Parkinson’s disease based on the patients’ needs (Created with BioRender.com).
These areas are targeted because they are part of a brain network involved in motor and cognitive (thinking-related) functions. When dopamine levels drop, faulty signaling in this network leads to the motor and non-motor symptoms of PD. You can think of this network like a group of students working together on a project: if one student is missing, it becomes harder to reach the goal. In the brain, dopamine is like the student who keeps the group focused and on task. Without this student, the whole system struggles. Similarly, without enough dopamine, the brain regions that depend on it become overactive, like students talking over each other and creating chaos. DBS helps by sending pulses to calm the overactive signals, like a teacher stepping in to guide a struggling student group. This rebalances the network and helps reduce the symptoms of PD [1].
DBS of the Vim helps reduce tremors, allowing the patient to flip pages or hold a pen. Similarly, DBS of the GPi improves muscle movements, helping patients get out of bed or hold a toothbrush. DBS of the STN improves slow and shaky movements, muscle stiffness, and posture-related symptoms [3]. This makes tasks like brushing teeth, flipping pages, and daily commuting easier and reduces the risk of falls. Overall, these treatments improve the quality of life for PD patients.
Surgeons target these specific brain areas using a thin wire called an electrode. First, they drill a small hole in the skull and then carefully insert the electrode into the brain. The patient is awake during the surgery but feels no pain. This is because the brain does not have the neurons that detect pain signals [1]. When the tip of the electrode reaches the targeted area, the surgeon applies electrical pulses. If the surgery is successful, the tremors will stop, which both the surgeon and the patient will notice right away in the operating room. While this immediate response helps confirm that the electrode is in the right spot, it is not the final result. After surgery, the electrode is connected to a small device called an implantable pulse generator (IPG), which is placed under the skin. The IPG makes and sends out electrical pulses continuously, many times every second. In the weeks after surgery, doctors carefully adjust the DBS settings to find the amount of stimulation that works best to reduce symptoms [4].
How Does Deep Brain Stimulation Work?
To understand how DBS works, it is important to remember the underlying problem in PD—dopamine-producing neurons in the substantia nigra begin to die. The substantia nigra communicates with other brain areas, such as the STN and GPi. Dopamine allows these areas to stay balanced in their activity, neither too active nor too quiet. But in PD, the loss of dopamine disrupts this balance, so the STN and GPi become overactive and start sending too many signals telling the brain to slow down or stop movement or that cause body shaking [1, 4]. DBS directly targets the STN or GPi, reducing their overactivity and restoring better motor function. Unlike these two areas, the Vim is not part of the motor network affected by dopamine, so it does not become overactive when dopamine levels drop [2].
DBS helps control brain overactivity by delivering high-frequency stimulation (HFS) to calm these areas down. This stimulation acts like a surgical cut, reducing the activity of the brain regions [4, 5]. Although it may seem like the electrical pulses would make these areas more active, HFS actually blocks signals from overactive brain areas and turns on nearby neurons that naturally work to calm brain activity [5, 6]. This shows how DBS can help relieve symptoms in patients with PD.
Benefits and Limitations of Deep Brain Stimulation
DBS offers many benefits for managing PD symptoms. First, DBS improves movement compared to medication alone. Studies show that patients who receive DBS have more time without slow movements, and they can perform daily activities better. Second, DBS causes minimal damage to surrounding cells and tissues. This leads to fewer unexpected side effects compared to thalamotomy. Third, DBS can be adjusted, which means it can be personalized for each patient [1].
DBS also has some limitations. First, there are risks linked with DBS. The most common problem after surgery is infection because placing electrodes in the brain and a device under the skin can introduce bacteria into the body. Second, DBS is less effective for non-motor symptoms of PD, such as mood changes, because it mainly targets brain areas that control movement. Third, regular check-ups are needed to adjust the electrical signals because symptoms can change over time. Since these adjustments require special medical centers, patients living far away from medical facilities may struggle to get care [1].
The Future of Parkinson’s Disease Research
Researchers are exploring stem cell therapies as a potential treatment for PD. Stem cells are basic cells that can divide and turn into specialized types like dopaminergic neurons. By making more dopamine, these cells may be able to restore brain function and relieve PD symptoms linked to dopamine loss. However, this therapy has some challenges. It is hard to control the type of neuron a stem cell turns into, which can lead to unexpected results. For example, stem cells can form tumors (abnormal tissue masses) that could lead to new health issues for patients [7].
Scientists are working on new methods to replace lost dopamine- producing cells and help them survive [7]. If these methods are successful, they may provide PD patients with a chance for improved motor function. This will offer a safer and less invasive treatment option compared to DBS. The future of medicine is full of exciting possibilities, and you could be a part of it!
Glossary
Dopaminergic Neurons: ↑ Brain cells that make and release dopamine, a chemical that helps control movement, emotions, and motivation. Problems with these neurons can lead to diseases like Parkinson’s disease.
Dopamine: ↑ A chemical in the brain that controls many brain functions like movement, motivation, pleasure, and reward. It also influences memory and mood. In Parkinson’s disease, dopamine levels are low.
Thalamotomy: ↑ An older surgery that destroys a small part of the thalamus, an area of the brain, to reduce movement symptoms—especially tremor—in Parkinson’s disease.
Levodopa: ↑ A medication that turns into dopamine in the brain, a chemical important for movement and other functions, helping raise its level.
Deep Brain Stimulation (DBS): ↑ A surgery that implants a device to send electrical pulses to brain areas to help control abnormal signals in Parkinson’s disease and other movement disorders.
Electrode: ↑ A thin wire surgically placed in a specific area of the brain to deliver electrical pulses. This helps control movement problems in diseases like Parkinson’s disease.
Implantable Pulse Generator (IPG): ↑ A small, battery-powered device surgically placed under the skin in the chest. It produces electrical pulses that help control overactive areas of the brain.
High-Frequency Stimulation (HFS): ↑ Regular current pulses at a high frequency (130–185 Hz) that induce inhibitory signals to regulate abnormal neuron activity.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by a grant from United Arab Emirates University, SUREPLUS (12M267).
AI Tool Statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
References
[1] ↑ Malvea, A., Babaei, F., Boulay, C., Sachs, A., and Park, J. 2022. Deep brain stimulation for Parkinson’s disease: a review and future outlook. Biomed. Eng. Lett. 12:303–16. doi: 10.1007/s13534-022-00226-y
[2] ↑ Hariz, M. and Blomstedt, P. 2022. Deep brain stimulation for Parkinson’s disease. J. Intern. Med. 292:764–78. doi: 10.1111/joim.13541
[3] ↑ Mandat, T., Koziara, H., Nauman, P., Tykocki, T., and Bonicki, W. 2011. Subthalamic nucleus deep brain stimulation in Parkinson’s disease. Biocybern. Biomed. Eng. 31:47–55. doi: 10.1016/s0208-5216(11)70018-3
[4] ↑ Benabid, A.-L., Wallace, B., Mitrofanis, J., Xia, C., Piallat, B., and Fraix, V., et al 2005. Therapeutic electrical stimulation of the central nervous system. C. R. Biol. 328:177–86. doi: 10.1016/j.crvi.2004.10.011
[5] ↑ Shehab, S., D’souza, C., Ljubisavljevic, M., and Redgrave, P. 2018. Activation of the subthalamic nucleus suppressed by high frequency stimulation: a c-Fos immunohistochemical study. Brain Res. 1685:42–50. doi: 10.1016/j.brainres.2018.01.034
[6] ↑ Benabid, A. L., Chabardes, S., and LeBas, J. F. (2009). “Subthalamic nucleus stimulation in Parkinson’s disease”, in Deep Brain Stimulation, eds. P. Bain, T. Aziz, X. Liu, and D. Nandi (Oxford: Oxford University Press), 3–10. doi: 10.1093/med/9780199543717.003.0001
[7] ↑ Lindvall, O. 2016. Clinical translation of stem cell transplantation in Parkinson’s disease.. J. Intern. Med. 27930–40. doi: 10.1111/joim.12415
