Abstract
Cancer is a major challenge for modern healthcare and, unfortunately, many people are confronted with this disease during their lives. Radiotherapy, which uses high-energy rays to destroy cancer cells, has been successfully used for many decades. However, traditional radiotherapy can only be used to treat one or a few cancer spots, and it damages the healthy tissues surrounding these spots. In this article, we introduce another treatment that uses radiation to beat multiple cancer spots in a very precise manner: targeted radionuclide therapy. You will get to know the “weapons” that kill cancer cells and how these weapons are delivered only to the cancer cells. Finally, you will learn about a powerful molecule that can be used as a “scout” to find the cancer cells and carry a “weapon” to kill those cells.
What is Cancer?
Cancer presents one of the greatest challenges in modern medicine. Cancer is a group of diseases that all share a common trait: cells start to divide rapidly and uncontrollably, forming masses that damage the function of vital organs. Radiotherapy, which is a type of treatment used to fight cancer by using high-energy rays, is an effective method that has been used for many years to kill cancer cells. However, radiotherapy damages more than just cancer cells. For example, by directing a beam of radiation at the cancer cells, the cancer cells in that location are killed, but there is also unintended damage caused to the surrounding healthy tissues that are hit by the radiation beam. Furthermore, radiotherapy cannot be used for cancer that has spread throughout the body.
What if doctors could harness the power of radiotherapy, delivering it more precisely to cancer cells while sparing healthy tissue, and simultaneously treating all spots of cancer throughout the body?
What are Radionuclides?
Before we can discuss radiation, we need to talk about the basic building blocks of everything around us: atoms. An atom has a nucleus made of neutrons and protons, surrounded by a cloud of electrons (Figure 1A). In a normal atom, the nucleus is stable, meaning that the number of neutrons and protons is in balance. Atoms with unstable nuclei are radioactive and are called radionuclides. Because the unstable atom wants to become stable, radioactive particles are released (Figure 1B).
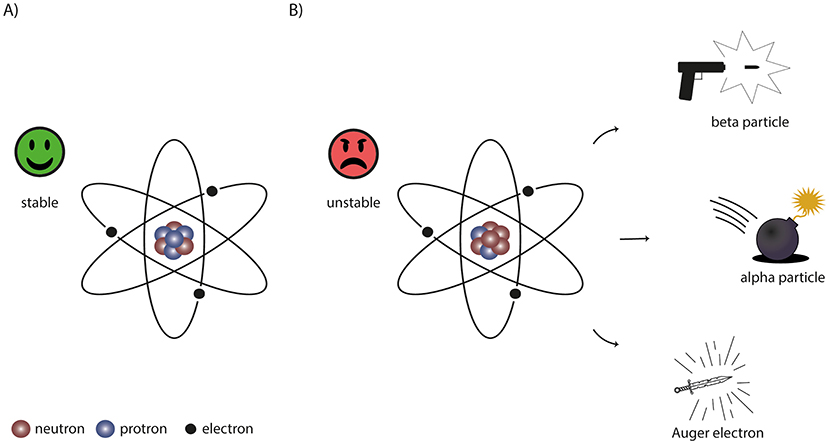
- Figure 1 - (A) A stable atom consists of neutrons and protons in the nucleus and an electron cloud around it.
- (B) An unstable atom, or radionuclide, emits a radioactive particle to become stable. There are three types of radioactive particles that can be used for radionuclide therapy: beta particles, working as “bullets”, alpha particles acting as “bombs”, and Auger electrons that work like “swords”.
Imagine the radioactive particle as a very tiny weapon that can kill a cancer cell. There are three different types of weapons (Figure 1B). One type of radionuclide shoots small “bullets” that can hit cells both nearby and further away, but the amount of damage is limited. These bullets are called beta particles. An example of a beta-emitting radionuclide is lutetium-177. A second radionuclide throws a “bomb”: it has a very high impact but only close by, therefore it only kills the neighboring cells. These bombs are called alpha particles. Alpha particles are emitted from radionuclides with very heavy nuclei, such as actinium-225. The final radionuclide acts as a deadly sword—it needs to be very close to the cell, preferably inside. This weapon is called an Auger electron. An example of an Auger-emitting radionuclide is indium-111.
Remember, we are calling these radioactive particles “weapons”, but we are actually talking about tiny particles that can be used to treat cancer. This treatment is called targeted radionuclide therapy. Now you understand the words “radionuclide” and “therapy”, but what does “targeted” mean? Well, if a doctor treats a patient directly with these weapons, they would not be able find their way to the cancer cells. “Targeted” means doctors make sure that these weapons are directed to cancer cells and not to healthy cells.
Targeting Radionuclides to Cancer Cells
Now let us take a closer look at a cancer cell. Like all cells, the outside of the cancer cell is “decorated” with many kinds of proteins. Some of these proteins are very abundant on cancer cells and hardly present on normal cells. These are called overexpressed proteins. Furthermore, some proteins on cancer cells can look slightly different compared with the same proteins on normal cells—these are called mutated proteins.
Scientists can make a molecule that specifically sticks to overexpressed or mutated proteins on cancer cells. In this way, they can target only the cancer cells and not the healthy cells. The radionuclide weapon of choice can be attached to this molecule, which allows scientists to target these weapons to the cancer cells (Figure 2A). This delivery molecule coupled to a radionuclide is called a radiotracer.
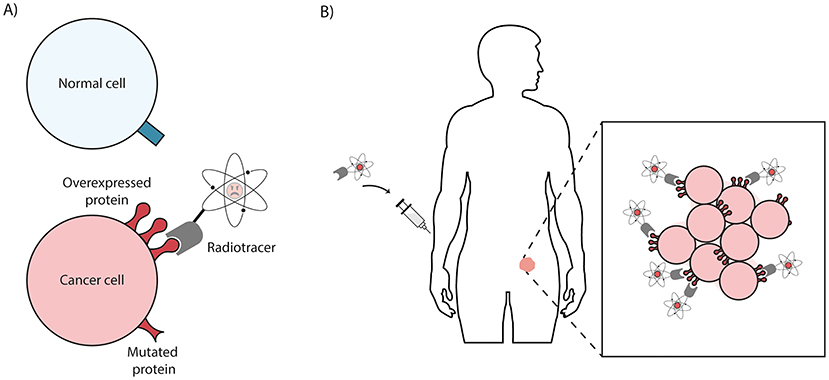
- Figure 2 - (A) A radiotracer is a radionuclide coupled to a delivery molecule (gray).
- The radiotracer only binds to overexpressed and mutated proteins on cancer cells (red) and not to normal cells (blue). (B) If the radiotracer is injected into the bloodstream of a patient, the radiotracer passes right through normal healthy tissues but sticks to cancer cells because they have the overexpressed or mutated proteins on their surfaces.
How does this process work? First, a radiotracer is prepared in a specialized laboratory. Then, it is injected into the bloodstream of a cancer patient by a nuclear medicine doctor. Within seconds, the radiotracer is pumped through the body via the blood circulation, and it travels through all the organs. When the radiotracer encounters healthy tissues, it just passes by. As soon as it discovers a cancer cell with the overexpressed or mutated protein on its surface, it holds on and sticks to the cancer cell (Figure 2B). Sometimes it can even be taken up by the cancer cells to become trapped inside. In the meantime, the radiotracer uses its weapons to shoot bullets, throw bombs, or strike with a sword. Ultimately, these weapons will severely damage cancer cells, causing them to die.
Example: Treatment of Prostate Cancer With Radionuclide Therapy
Some radiotracers are already being successfully used to treat cancer patients. One example is a radiotracer that specifically targets prostate cancer cells. The prostate is a gland found only in males, located just below the bladder. Prostate cancer is caused by uncontrolled growth of cells in the prostate gland. Prostate cancer cells overexpress a protein called PSMA on their surfaces. PSMA is barely expressed on healthy tissues, therefore it is an excellent target for treating prostate cancer patients with radionuclide therapy [1].
Scientists have developed radiotracers that strongly bind to PSMA. However, before this treatment could be used in patient care, scientists and doctors had to prove that the treatment is safe and effective. This was done in two types of studies.
First, in laboratory studies, scientists showed that the PSMA radiotracer strongly binds to PSMA on prostate cancer cells in a culture dish. Second, the radiotracers were tested in laboratory animals to see: (1) if they could be safely injected into the bloodstream, (2) if they bind to the cancer cells, and (3) if they kill the cancer cells. These experiments showed very promising results, so scientists moved on to the next phase of testing.
Next, in human studies, one group of patients received the standard treatment for prostate cancer, while the other group of patients received the standard treatment plus PSMA-targeted radionuclide therapy. Scientists and doctors followed these patients over time and found that patients treated with PSMA-targeted radionuclide therapy live longer and healthier lives [2]. However, as the patients were already very ill at the start of treatment, it was not possible to fully cure them. Now, large clinical studies have begun, to investigate if prostate cancer patients can be cured when the treatment starts earlier after diagnosis. These studies take a lot of time, but the first results look very promising.
While treatment with radiotracers holds great promise for prostate cancer patients, the presence of the PSMA protein is crucial for PSMA-targeted radionuclide therapy to be effective. Therefore, patients whose cancer cells do not have PSMA should not be unnecessarily treated—they should start a different kind of therapy. How can doctors accurately distinguish between patients who may or may not benefit from PSMA-targeted radionuclide therapy, ensuring the best treatment option for each patient?
Radionuclide Imaging to Select the Right Patients
Here is where this story gets even more exciting. Theranostics is a combination of two important words: diagnostics (diagnosing an illness) and therapeutics (treating an illness). To know if the patient’s prostate cancer has PSMA, doctors can use the same radiotracer but replace the “weapon” radionuclide with a “scout” radionuclide that does not harm the cells. Scout radionuclides can be detected with a specialized scanner that creates a picture to locate the radioactivity in the body. This technology is called radionuclide imaging. If doctors see a radioactive signal in regions where the cancer cells are, then they can treat the patient with PSMA-targeted radionuclide therapy. Interestingly, the same imaging technology can also be used after treatment, to evaluate whether the therapy worked or whether the patient needs another round of treatment (Figure 3).
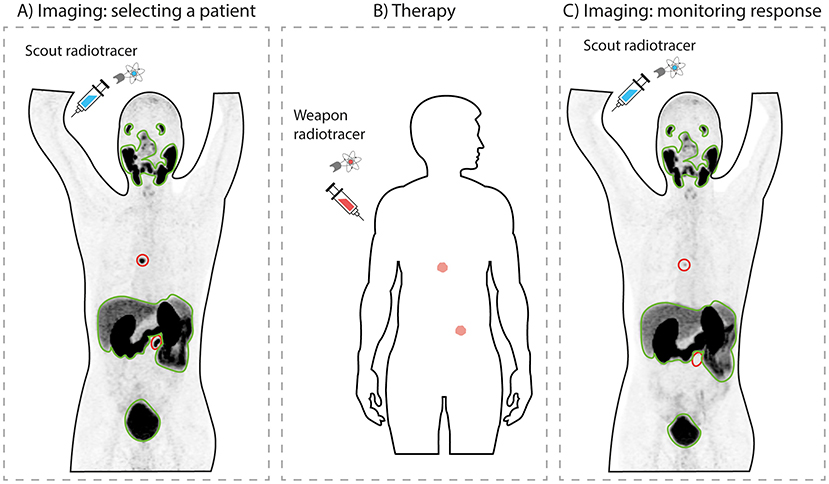
- Figure 3 - Theranostics.
- (A) First, a scout radiotracer is used to scan a patient to determine whether cancer cells have PSMA. The darker the color, the more radiotracer is present. Here, cancer sites are the two black spots, circled in red. Healthy organs (outlined in green) also take up some radiotracer. (B) Since this patient’s cancer cells have PSMA, he can be treated with a weapon radiotracer. (C) After treatment, a scout radiotracer is used again, to monitor the patient’s response. This patient had a good response, since the black signal declined in the cancer sites indicating that the cancer is shrinking (image modified from Privé et al. [3]).
So, theranostics is like having a superhero molecule to find and fight cancer, making treatments more precise and effective. Currently, many more radiotracers are being developed to treat patients with various other cancer types. We are excited about the future of theranostics as it harnesses the power of radiation to battle cancer!
Glossary
Radiotherapy: ↑ A type of treatment that is used to fight cancer by using high-energy rays.
Radionuclides: ↑ Unstable atoms that can become stable by releasing tiny radioactive particles.
Radioactive Particles: ↑ Tiny particles that can damage cells. These particles are released by unstable atoms.
Targeted Radionuclide Therapy: ↑ A kind of medical treatment that uses tiny radioactive particles connected to a delivery molecule to target and fight cancer cells.
Overexpressed Protein: ↑ A protein that is present in larger-than-normal amounts on cells. This is often the case for cancer cells.
Mutated Protein: ↑ A protein that has changed due to a mistake in the DNA, which is often the case for cancer cells.
Radiotracer: ↑ A molecule that carries a radionuclide to a specific cell.
Theranostics: ↑ Using a powerful molecule that can help doctors to both find a disease (diagnostics) and treat the disease (therapeutics).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
[1] ↑ Sandhu, S., Guo, C., and Hofman, M. S. 2021. Radionuclide therapy in prostate cancer: from standalone to combination PSMA theranostics. J. Nucl. Med. 62:1660–8. doi: 10.2967/jnumed.120.243295
[2] ↑ Sartor, O., de Bono, J., Chi, K. N., Fizazi, K., Herrmann, K., Rahbar, K., et al. 2021. Lutetium-177–PSMA-617 for metastatic castration-resistant prostate cancer. N. Engl. J. Med. 385:1091–103. doi: 10.1056/NEJMoa2107322
[3] ↑ Privé , B. M., Peters, S. M. B., Muselaers, C. H. J., van Oort, I. M., Janssen, M. J. R., Sedelaar, J. P. M., et al. Lutetium-177-PSMA-617 in low-volume hormone-sensitive metastatic prostate cancer: a prospective pilot study. Clin. Cancer Res. (2021) 27:3595–601. doi: 10.1158/1078-0432.Ccr-20-4298