Abstract
Batteries are everywhere in our lives—from our phones and watches to cars and military equipment. Lithium ion batteries (LiBs) are a rechargeable kind of battery often used in common electronic devices. Researchers are working hard to improve batteries, so they can be used for longer without recharging and so they can store more energy—perhaps even energy from wind or solar sources that we can use to power our homes and businesses. Recent research has shown that LiBs can be improved by using extremely tiny materials with special properties, called nanomaterials. When they are used in LiBs, nanomaterials can increase the amount of energy that can be stored and decrease the amount of time it takes to recharge. Nanomaterials can also extend the life of LiBs. In this article, we will explain how LiBs work and how nanomaterials might be used to improve their performance.
Batteries are Energy-Storage Devices
Fossil fuels, such as coal, crude oil, and natural gas, have long been the world’s primary energy source. However, these fuels will eventually run out and, in the meantime, they are causing widespread pollution of the environment, through production of carbon dioxide (which contributes to global warming) and other dangerous substances. In the face of this challenge, there is increasing pressure to use environmentally friendly, renewable energy sources like wind and solar power. However, these sources do not produce power in a steady way that could support the world’s energy needs. Communities need power all day and night, but the sun does not shine all the time and the wind does not blow constantly—so, there must be a way to store energy from renewable sources, so that it can be released as needed. Electrochemical energy-storage devices are one solution. These devices work by storing electricity through chemical reactions that happen inside them. When the sun shines or the wind blows, these devices capture the energy and keep it in a chemical form that can be turned back into electricity through additional chemical reactions whenever people need it, like at night or when the air is still [1]. There are various kinds of electrochemical energy-storage devices (Figure 1), which can provide dependable, long-term backup power for households, companies, data centers, and other needs.

- Figure 1 - There are various types of electrochemical energy-storage devices, all of which store and release energy through chemical reactions that happen inside them.
- While batteries are the most well-known of these devices, fuel cells, which generate electricity by combining hydrogen and oxygen to produce water and electrical power without needing to be recharged, and supercapacitors, which store electrical energy on the surface of materials and can charge and discharge much quicker than batteries (but hold less energy), are other examples. Reproduced with permission from [1]. Copyright 2020, MDPI.
Batteries: Single-Use and Rechargeable
Batteries are probably the most well-known electrochemical energy- storage devices. A battery works as a kind of container that stores energy within the chemical compounds inside it, until the energy is needed. The chemical energy is converted into electric energy when the battery is used. Batteries can be classified as primary or secondary batteries. Primary batteries are intended to be used just once before being thrown away, while secondary batteries can be recharged and used repeatedly. Most primary batteries contain substances such as zinc and carbon, and they cannot be recharged because the chemical processes that happen inside them are irreversible. Primary batteries are sometimes called dry cells because most of them do not contain any liquids—just a paste-like substance. On the other hand, rechargeable or secondary batteries, such as lead-acid, nickel-cadmium, and lithium-ion batteries (LiBs), can be recharged several times and used repeatedly, since the reactions that happen inside them can be electrically reversed. There are multiple types of secondary batteries—some of them, like car batteries, have wet cells that contain a fluid that helps the electricity to move through them, while others, like the LiBs that power many portable electronic devices, have dry cells. Rechargeable batteries are becoming preferable due to the demands of the modern world and the rising number of portable devices [2].
How Do Rechargeable LiBs Work?
LiBs are found in many everyday household items like flashlights, cameras, toys, medical equipment, portable electronics, and security systems (Figure 2). These batteries can store a lot of energy for their size, hold on to that energy for a long time, and be successfully recharged many times. But have you ever wondered how they work? Imagine the battery as a sandwich. In this sandwich, there are two pieces of bread on either side called electrodes, one is positive (called the cathode), and the other is negative (called the anode). The “filling” between these pieces of bread includes a special material called a separator and a liquid called the electrolyte (Figure 3A).
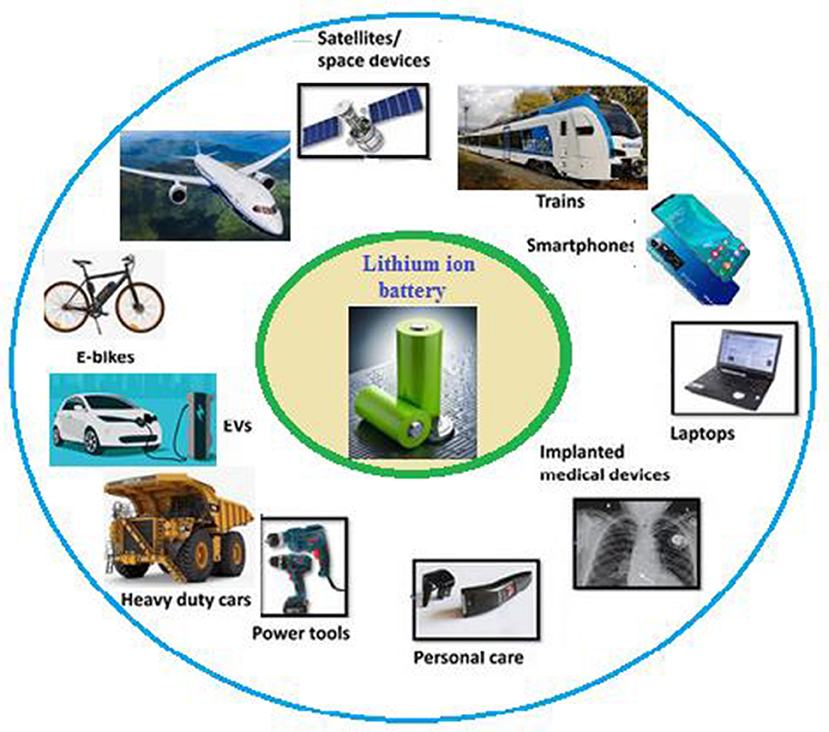
- Figure 2 - LiBs are used as power sources in many types of devices, such as smartphones, laptops, watches, torch lights, military equipment, aircraft, electric vehicles, and more.
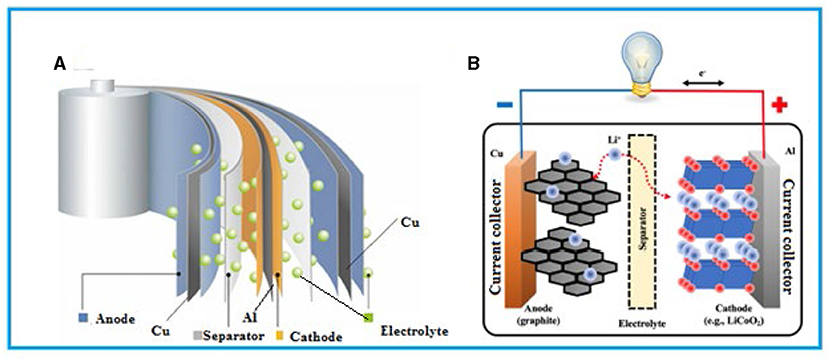
- Figure 3 - (A) The inside of a LiB looks kind of like a sandwich.
- The two slices of “bread” are the anode and cathode. They are kept apart by the “filling”—a special material called the separator, which allows ions to flow through but stops the anode and cathode from touching. (B) Within the battery, the lithium ions (Li+) move from the anode through the separator to the cathode when power is needed and back to the anode when the battery is recharged. Movement of ions from the anode to the cathode makes electricity, in the form of electrons, move through wires, which powers the device [4].
The separator is a very thin layer that keeps the positive and negative sides from touching each other, which is super important for safety and for making sure the battery works properly. The electrolyte is a special kind of liquid that helps lithium ions (tiny charged particles of the metal lithium) move back and forth between the two pieces of bread when the battery is being used or charged [3]. In batteries, the separator material is made from a membrane with many microscopic holes in it. These membranes can be made of various substances, and the ones used in LiBs are chosen for their mechanical strength, chemical resistance, and heat stability.
When you use your phone, for example, and the battery is releasing power, lithium ions move from the negative side to the positive side through the electrolyte. This movement creates electrical energy that powers your device (Figure 3B). When you charge your phone, you are pushing those lithium ions back to the negative side, getting the battery ready to work again.
The materials for the electrodes are also chosen based on their properties. The cathode must be positively charged and really good at attracting electrons, while the anode must be negatively charged and great at releasing electrons. This difference is why electricity flows in the battery, and why your phone turns on and works. Common materials used for the electrodes are graphite (a form of carbon) for the negative side and special metals like lithium cobalt oxide for the positive side.
The other important part of a battery is the current collector. Its main purpose is to transfer electrons from the electrode materials to an external circuit and then to the device (example light bulb). The current collector also provides mechanical and conductive supports for the electrode material. Various metals, including aluminum and copper, are commonly used in current collectors.
While LiBs are smaller and lighter than other types of rechargeable batteries, they still have their drawbacks. They can overheat, catch fire, or even explode if they are damaged or if they are charged or stored improperly. They are also not powerful enough to meet the large-scale energy storage demands required for a complete transition away from fossil fuels. Storing energy from wind and solar sources, which are variable and sometimes produce more power than we can use immediately, requires batteries that can hold a lot of energy and release it steadily when needed. One way to make batteries more efficient, safer, and capable of handling the energy storage challenges of renewable energy sources involves using special materials called nanomaterials.
What are Nanomaterials and How Can They Improve Batteries?
Nanomaterials are new substances that have become increasingly important in recent years. They are special because their basic parts, called nanoparticles, are incredibly small, about a billionth of a meter in size. Think of nanoparticles like building blocks that can be arranged in various ways to create nanomaterials. The extremely tiny size of nanoparticles gives nanomaterials unique properties like being able to conduct electricity, react with other substances in interesting ways, or even interact with light and electricity differently than “normal” materials [5]. This makes nanomaterials useful in lots of fields like medicine, electronics, and chemistry.
One big area in which nanomaterials are making a difference is in LiBs. First, the extremely small size of nanomaterials reduces the distance that lithium ions must travel within the battery. This makes the battery work more efficiently, allowing it to store more energy and charge faster. Nanomaterials also contribute to the battery’s durability and safety. Their ability to tolerate changes in structure during the battery’s charging and discharging cycles helps prevent the battery from getting damaged over time. This is crucial for maintaining the battery’s performance and extending its lifespan. One of the biggest advantages of nanomaterials is their large surface area compared to their volume. A large surface area improves the interaction between the battery’s electrodes and the electrolyte. As a result, lithium ions move more freely and quickly, enhancing the battery’s ability to charge and discharge rapidly.
Nanomaterials are used in various parts of LiBs, including the electrodes and the electrolyte. In the electrodes, nanomaterials help store more lithium ions, which increases the battery’s energy storing capacity. When used in the electrolyte, nanomaterials improve the movement of lithium ions, making the battery more efficient. Additionally, coatings made from nanomaterials can be applied to the separators, which can enhance the battery’s safety by preventing short circuits and other damage [6].
Building the Batteries of the Future
As good as this sound, using nanomaterials in LiBs also comes with some challenges. One problem is that nanomaterials can react in ways we do not want them to, especially because they have a really big surface area for their size. This means they can easily react with other substances, like the electrolyte, inside the battery. These unwanted reactions can create layers of buildup on the nanomaterials that slow down how fast lithium ions can move around, preventing the battery from lasting as long or holding as much power as it should. To fix these issues, scientists are trying to make nanomaterials more stable and they are also experimenting with special additives or protective coatings to stop these unwanted reactions from happening. Also, the way nanomaterials are put together inside the battery needs to be carefully thought out, to make sure batteries work as best as they can. The aim is to figure out both how to use nanomaterials effectively and how to produce them on a large scale.
In conclusion, using nanomaterials allows engineers to adjust the components of LiBs—and other types of electrochemical energy storage devices—on a tiny scale. Nanoparticles might improve battery capacity, charging speed, safety, and lifespan, leading to smaller, more powerful, and longer-lasting LiBs. Researchers must continue to tackle the remaining challenges, because electrochemical energy storage devices are becoming more important every day! Not only will better batteries make our lives easier by powering all our devices, but powerful batteries can also help us store the energy produced from renewable sources. This means that the batteries of the future could help countries transition away from burning fossil fuels. So, you can see that improving batteries not only makes our daily lives easier—it might actually help save the planet!
Glossary
Electrochemical Energy Storage: ↑ A way of keeping energy in chemical form inside batteries and other storage systems, which can then be turned into electricity when we need to power electronics or other devices.
Electrode: ↑ A part of a battery where electricity goes in or out, helping to connect the battery’s power to the things it runs.
Separator: ↑ A special layer within a battery that keeps the positive and negative sides apart to prevent short circuits, while still allowing electricity to flow through.
Electrolyte: ↑ A substance that contains ions and can conduct electricity, often used in batteries to help move an electrical charge between the electrodes.
Ions: ↑ Tiny particles that carry an electric charge, either positive or negative, and are important in creating electricity in things like batteries.
Nanomaterials: ↑ Nanomaterials are super tiny materials, much smaller than we can see, used in technology to make products like batteries work better and do more things.
Nanoparticles: ↑ The incredibly small building blocks of nanomaterials. Nanoparticles often have special properties due to their tiny size, and can be used to improve products, including batteries and medicines.
Surface Area: ↑ The total space on the outside of an object, like measuring all the sides of a box to see how much it can cover.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
[1] ↑ Siwal, S. S., Zhang, Q., Devi, N., and Thakur, V. K. 2020. Carbon-based polymer nanocomposite for high-performance energy storage applications. Polymers 12:505. doi: 10.3390/polym12030505
[2] ↑ Schmidt-Rohr, K. 2018. How batteries store and release energy: explaining basic electrochemistry. J. Chem. Educ. 95, 1801–1810. doi: 10.1021/acs.jchemed.8b00479
[3] ↑ Niu, H., Wang, L., Guan, P., Zhang, N., Yan, C., Ding, M., et al. 2021. Recent advances in application of ionic liquids in electrolyte of lithium ion batteries. J. Energy Storage 40:102659. doi: 10.1016/j.est.2021.102659
[4] ↑ Wahl, M. S., Spitthoff, L., Muri, H. I., Jinasena, A., Burheim, O. S., and Lamb, J. J. 2021. The importance of optical fibres for internal temperature sensing in lithium-ion batteries during operation. Energies 14:3617. doi: 10.3390/en14123617
[5] ↑ Bruce, P. G., Scrosati, B., and Tarascon, J. 2008. Nanomaterials for rechargeable lithium batteries. Angew. Chem. Int. Ed. 47, 2930–2946. doi: 10.1002/anie.200702505
[6] ↑ Hur, J. 2022. Nanomaterials for ion battery applications. Nanomaterials 12:2293. doi: 10.3390/nano12132293
