Abstract
When you feel cold, you might put on a wool sweater to stay warm. But on a rainy day, you might want to make your outer layer a waterproof shell, like a rain jacket. In both cases, you are protecting yourself from the surrounding environment (cold or rain), and the clothes you choose will depend on the weather outside. Now, what if we wanted to protect very tiny particles, called nanoparticles, in a similar way? Instead of clothing, we have to use a coating—a layer of another material that protects the particles from their environment. But it would be even better if this coating could be “smart” and change in response to the surrounding environment! In this article, we will tell you how a smart coating made out of silica (sand) can protect nanoparticles more efficiently, so that they can be used in many different applications, like delivering medicines to where they are needed in the body.
Why Do Tiny Particles Need Coats?
Have you ever heard of the word “coating”? A coating is a protective layer that you put on something to keep it safe and protected from the surroundings. For instance, you might put a coat of sunscreen on your skin to protect it from the sun’s rays, or a layer of frosting on a cake to protect it from drying out (and to make it more delicious). These are things that we can easily see and touch with our eyes and hands. But did you know that scientists can also use coatings for things that are way too tiny to see, like super tiny particles called nanoparticles [1]?
Nanoparticles are so small that they cannot be seen by our eyes, but they play incredibly important roles in medicine, electronics, and even in food. For example, in medicine, nanoparticles can help deliver drugs exactly where they are needed in the body. Nanoparticles can be made of several types of substances, including metals like silver or gold; special plastics; or even tiny, hollow structures called carbon nanotubes.
So, why do these tiny particles need coats? Nanoparticles can be very delicate and easily damaged (Figure 1A). By giving them a tough outer layer, scientists can protect them from being damaged, making them stronger and more useful (Figure 1B). One type of coating that scientists are super excited about is made of silica. Silica coats can protect nanoparticles like a superhero’s armor [2]. For example, when exposed to heat, like a sunny day, these coatings can act like a protective shield—the same way you might put on a sunhat or sunscreen to stay safe from the sun.
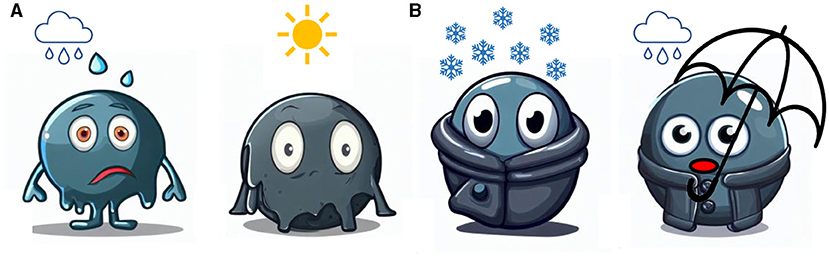
- Figure 1 - (A) Nanoparticles are often very sensitive and can break down under environmental conditions like too much moisture or too much heat.
- (B) Substances like silica can be used to create “coats” for nanoparticles, to protect them from their surroundings and help them to do their jobs better.
What is Silica?
Think about sand at the beach. It is made up of tiny pieces called grains. Now, if you could see the building blocks of sand grains—the molecules that make them—you would see silicon dioxide, the scientific term for silica [3]. Silica is found all over in nature, in things like rocks, plants, and even in bacteria. For example, bacteria have some very clever ways to put a coat of silica on themselves, which helps them to adapt and thrive in challenging environments, such as extreme heat or other hazardous conditions. There are also tiny living organisms called diatoms, which are a type of plankton that have intricate shells made of silica, and silica sponges, which create amazing shapes out of this substance. We got our inspiration to make silica coats for nanoparticles based on these amazing natural creations.
But why does silica make such a great coating? One reason is that silica generally does not undergo chemical reactions with other molecules, which makes it stable. But even better, silica can team up with other substances to make materials with new, useful properties.
Making Silica Coats “Smart”
Our curiosity about silica led us to explore ways to enhance silica coatings, giving them remarkable abilities. We think of these as “smart” coatings because they can change depending on the environment. Imagine Iron Man’s suit, which just vanishes when he needs it to. In the same way, smart silica coats can be removed when we want them to be, revealing the nanoparticles underneath.
Why would we want to remove a silica coat from a nanoparticle? Well, for example, imagine a nanoparticle designed to deliver a medicine. The silica coat acts as a protective layer during transport, ensuring the medicine reaches its destination intact. However, once the nanoparticle reaches its target, we might want to remove the silica coat to allow efficient release of the medicine.
But, removing the silica coats from nanoparticles is usually not easy, because they are so stable. It typically requires the use of a powerful solution known as hydrofluoric acid (HF). HF is an extremely dangerous substance, posing risks not only to the coated nanoparticle but also to the scientists handling it—and HF definitely cannot be used to remove coats from nanoparticles in the human body! So, how do we safely remove the coats from nanoparticles so that they can do their jobs? We decided to try to develop a smart silica coating that can be easily removed without using any harmful solutions, such as HF.
Creating Acid-Sensitive Smart Coats
Out of all the exciting ways we could choose to make our silica coatings smart, we decided to look at pH. pH measures the acidity or basicity of a substance. Lemon juice tastes sour because it has a low pH, meaning it is acidic. On the other end of the scale, substances with a high pH are basic, like baking soda or ammonia. Some liquids, like milk or pure water, are neither acidic nor basic—they are neutral.
We decided to make a smart silica coating that changed in response to changes in pH. When adding a drop of an acidic substance like lemon juice, poof, the silica coating disappears! This is an important goal because it allows us to precisely control the behavior of the coating based on the environmental conditions. This disappearance is due to the disintegration of the silica at low pH, revealing the core nanoparticles. For example, inside the cellular compartments, such as endosomes and lysosomes, the environment tends to be more acidic. In the context of drug delivery using nanoparticles, the ability of the coating to respond to changes in acidity inside these cellular compartments is valuable for targeted and controlled release of medicines.
For our experiment, we started with spherical nanoparticles made out of a substance called polystyrene (PS), which is a kind of plastic. First, we practiced coating these nanoparticles with a regular silica coating, without any special changes. Before adding the silica, we coated the polystyrene nanoparticles with a sticky helper substance that helps the silica stick to them like glue. Then we looked at these coated particles using a powerful microscope called a transmission electron microscope (TEM). This allowed us to see these tiny particles, which are 1,000 times smaller than the diameter of a hair. When we looked at the images, we saw that most particles had a coating that got thicker as we used more silica, and the coating was smooth and even (Figure 2). The coated nanoparticles had the same sphere shape as the uncoated particles.
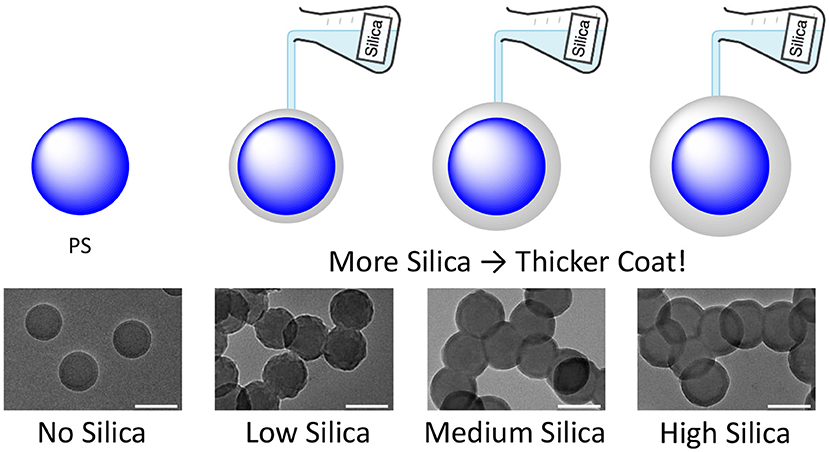
- Figure 2 - A silica coating can be used to build a shield around nanoparticles.
- The more silica we add, the thicker the coating becomes. The bottom images show what the nanoparticles looked like using TEM.
Once we knew how to make this regular silica coating on polystyrene nanoparticles, we wanted to make the coating smart. So, we added a new compound to our mixture, which we will call pH-silane, for simplicity. This compound is important for making the silica coating responsive to pH changes. We mixed pH-silane, the silica coating, and our nanoparticles together. When we looked at the nanoparticles using TEM, they looked different. Instead of a smooth, even silica coating, our nanoparticles looked like raspberries (Figure 3A)! We also checked to make sure the pH-silane was incorporated into the silica coating, and it was.
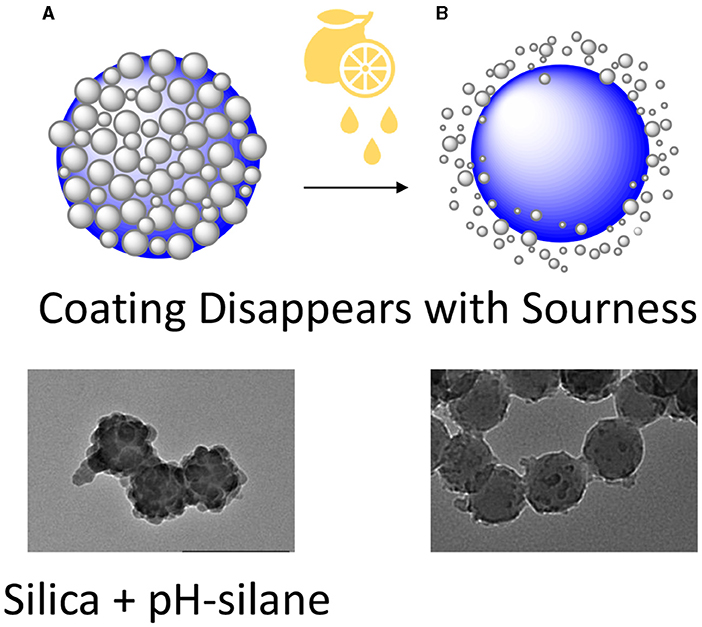
- Figure 3 - To create a “smart” coating for nanoparticles that is sensitive to low pH, we combined nanoparticles, silica, and a substance called pH-silane.
- (A) These nanoparticles looked different from those coated with silica alone—they had a bumpy, raspberry-like appearance. (B) When we added an acidic solution to these particles, their protective coats disintegrated. This told us we had successfully created “smart” nanoparticles that “took their coats off” when the pH was low.
Our Nanoparticles Took Their Coats Off!
Then came the fun part—testing! We put our particles in water with different pH levels. We tried pH 5.0 (acidic) and pH 7.4 (the neutral control). We watched closely to see how the particles changed. In the acidic solution, the special silica coating started to disappear (Figure 3B). But at neutral pH, the coating stayed strong. When we checked other nanoparticles that did not have the pH-silane mixed with silica, their coatings did not change at all at pH 5.0. This means that our new particles are “smart” and can lose their coats when the pH changes.
Why is This Research Important?
In our exciting journey, we developed a new and super cool technique to remove a protective silica coating from nanoparticles. Our work is an important step toward creating “smart” nanoparticles that can respond to changes in their environments. These smart silica coatings can both protect the nanoparticles and help them to work better. In our case, coatings that change in response to pH can help medicines work better by ensuring controlled and targeted release. For instance, inside certain cellular compartments where the environment is more acidic, the smart silica coating can facilitate the precise release of therapeutic agents, enhancing the effectiveness of drug delivery.
Other smart nanoparticles may be designed with coats that allow them to glow in the dark, or even release tiny surprises! We are at the beginning of a big adventure with these smart coatings, and, in the future, they could help solve many important problems in the world of science and medicine. So, our research is not just about tiny particles—it is about making things smarter and better for everyone!
Glossary
Nanoparticles: ↑ Tiny particles that are extremely small, often at the scale of nanometers (billionths of a meter).
Silica: ↑ A compound made up of silicon and oxygen, often found in nature as quartz or sand. It is used in various forms in technology and industry.
pH: ↑ A measure of how acidic or basic a solution is. It ranges from 0 to 14, with lower values being acidic, higher values being basic, and 7 being neutral.
Polystyrene: ↑ A type of plastic that can be formed into various shapes. In this context, it’s used as a model or template for creating silica coatings on nanoparticles.
Transmission Electron Microscope (TEM): ↑ A powerful microscope that uses electrons instead of light to visualize extremely small objects, providing high-resolution images.
pH-silane: ↑ A special compound designed to make silica coatings responsive to changes in pH.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
AI Tool Statement
During the preparation of this work, the author used AI-assisted technologies to polish the text and enhance readability. After using this tool, editorial revisions were integral to the development of the manuscript. The author thoroughly reviewed and edited the content as needed and takes full responsibility for the accuracy, originality, and compliance with the journal’s standards.
Acknowledgments
Liga Portuguesa Contra o Cancro Terry/Fox Research Grant. This work was financed by national funds from FCT—Fundação para a Ciência e a Tecnologia, I.P., in the scope of the project UIDB/04565/2020 and UIDP/04565/2020 of the Research Unit Institute for Bioengineering and Biosciences-iBB and the project LA/P/0140/2020 of the Associate Laboratory Institute for Health and Bioeconomy—i4HB.
Original Source Article
↑Lima, M., Avó, J., Berberan-Santos, M. N., Crucho, C. I. C. 2022. pH-responsive silica coatings: a generic approach for smart protection of colloidal nanoparticles. ACS Appl.NanoMater. 5:9460–8. doi: 10.1021/acsanm.2c01723
References
[1] ↑ Schubert J., Chanana M. 2018. Coating matters: review on colloidal stability of nanoparticles with biocompatible coatings in biological media, living cells and organisms. Curr. Med. Chem. 25:4553. doi: 10.2174/0929867325666180601101859
[2] ↑ Wahid, A. A., Doekhie, A., Sartbaeva, A., Elsen, J. M. 2019. Ensilication improves the thermal stability of the tuberculosis antigen Ag85b and an Sbi-Ag85b vaccine conjugate. Sci. Rep. 9:11409. doi: 10.1038/s41598-019-47657-9
[3] ↑ Croissant, J. G., Butler, K. S., Zink, J. I., Brinker, C. J. 2020. Synthetic amorphous silica nanoparticles: toxicity, biomedical and environmental implications. Nat. Rev. Mater. 5:886. doi: 10.1038/s41578-020-0230-0