Abstract
Interfaces are all around us. A fluid interface is the boundary between two immiscible fluids, and a great example is oil and water. They do not mix. The formation of these interfaces is crucial for daily life. You cannot always see them, but you can encounter interfaces everywhere. They can be in soap, in ice cream and in your body. In this article, you will learn what an interface is, why you need them in your daily life, and how interfaces can be created. Or to be more specific, how you can create your own interface using special molecules called emulsifiers.
What Is an Interface?
Many of the substances we encounter in everyday life can be classified as fluids, which are liquids or gasses that can flow. Some common examples of fluids include water, oil and air. Some fluids can be easily mixed together, but some fluids are immiscible, which means they cannot be mixed—like water and oil. An interface, is the boundary between two immiscible fluids. Fluid interfaces are everywhere in our daily lives, but we cannot always see them. For example, we encounter interfaces in the bathroom, when we create a nice foam using shampoo or soap. Foods such as ice cream and mayonnaise cannot exist without the creation of interfaces [1]. The same hold for us, as the human body cannot function without interfaces [2]. Interfaces occur between liquids (like oil and water) as well as liquids and gasses (like air and water).
The concept of an interface is actually quite simple. You can create your own interface by filling a transparent beaker or glass one-fourth full with water. Then, carefully pour some oil (for instance olive oil) on top, and wait. Over time, you will see a layer of water at the bottom and a layer of oil on top. This experiment shows that water and oil are immiscible. Most oils have a lower density than water (10 mL of oil is lighter than 10 mL of water) so, due to the immiscibility and to gravity, the oil layer floats on top of the water. The boundary between the oil and the water is called the interface.
Like oil and water, the mixing of any immiscible liquids creates interfaces. This is also true for gas-liquid mixtures, such as air and water: the two phases are immiscible; they do not dissolve in each other the way sugar dissolves in water. Due to immiscibility, we can create spherical droplets of one fluid within another fluid. In case of oil-water interfaces, we call this an emulsion; and in case of air-water interfaces, we call it a foam. Foam and emulsions can be used to give structure to certain products, like ice-cream (Figure 1). Without the foam and emulsion, ice cream would be a frozen brick without a nice texture.
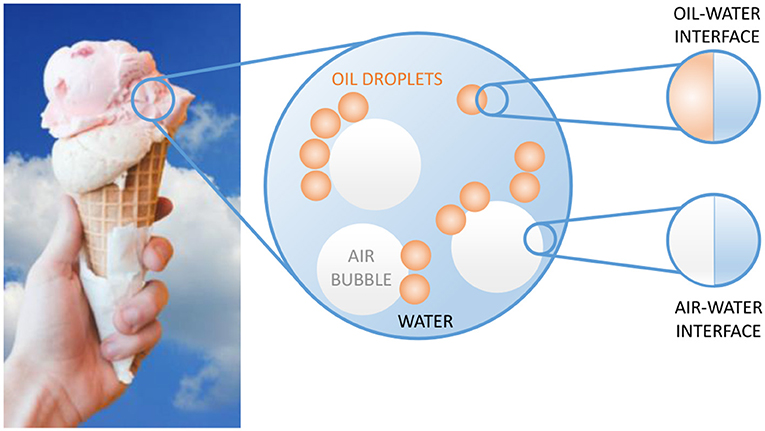
- Figure 1 - Ice cream: a well-known encounter with interfaces.
- Ice cream is a tasty but complex food product. It contains oil droplets and air bubbles. The oil droplets are surrounded by water, and the boundary between the oil droplets and the water is called the oil-water interface. The same holds true for air bubbles in ice cream—the boundary between the air and the water is called the air-water interface.
How Can We Create More Interfaces?
Remember that we told you that interfaces are everywhere, but you cannot always see them? The oil-water interface that you created in a glass is clearly visible. But there are also many interfaces that you cannot see. For instance, when you lather up with soap in the shower or when you wash your hands, you create a foam. This foam contains millions of air bubbles, and each of these air bubbles is surrounded by water. Air-water interfaces are thus present. The millions of small air bubbles that make up the foam contain a very large amount of interfaces.
We can visualize this with an experiment (Figure 2). For this experiment, you need two plastic bottles with caps. Fill half of Bottle 1 with tap water. Add the same amount of water plus a few droplets of soap to Bottle 2. You already know that an interface is present between the air and the water. Now, close the two bottles and vigorously shake them for 15 s. What do you see?
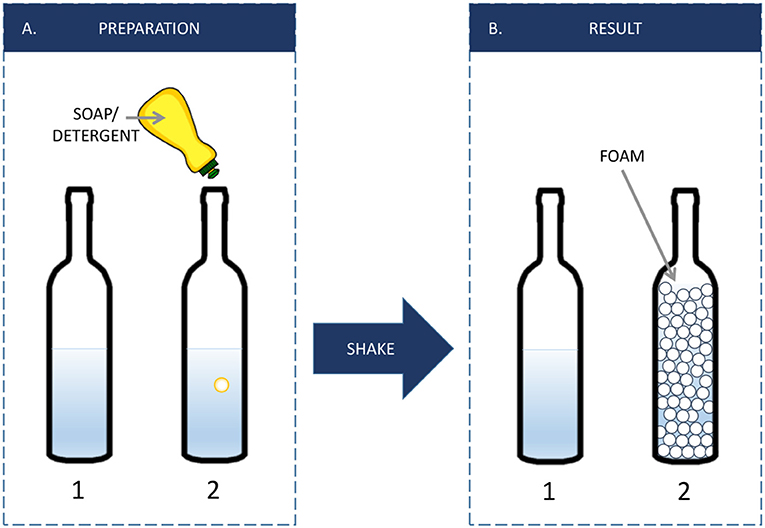
- Figure 2 - (A) We can use soap to create more fluid interfaces.
- Fill two bottles half way with water. Add a few drops of soap to one of the bottles. Shake both bottles—what happens? (B) In bottle 1, nothing happens, but if you shake the bottle with the soap (bottle 2) long enough, you will increase the amount of interface by creating a foam, consisting of many air bubbles. This is because of the presence of emulsifiers in the soap.
In Bottle 1, you probably do not see much change. In Bottle 2, you probably see a big, white layer—a foam. The foam is made of many air bubbles, each of which is surrounded by an interface. So, in Bottle 2, you created many air bubbles, and thus created much more interface than exists in Bottle 1. Amazing is it not?!
Why Is no Foam Formed in Bottle 1?
So, why did we only create air bubbles in Bottle 2, not in Bottle 1? To explain this observation, we must zoom in on the interface between air and water. Individual water molecules are so small that we cannot see them even with powerful microscopes. For instance, one liter of water contains 3,343,000,000,000,000,000,000,000 water molecules. Can you imagine how small a water molecule must be? Now back to the interface: water molecules like to be surrounded by other water molecules—this is when they are in their most relaxed state. The water molecules at the air-water interface are only partly surrounded by water, as they are also surrounded by air (Figure 3A). This is stressful and undesirable for the water molecules; they do not want to stay in this situation. Now imagine shaking Bottle 1: we try to generate more interfaces by creating air bubbles. This is unfavorable for the water molecules present at the new interfaces, so the water molecules will go back to their most favorable situation, which is being surrounded by water. So, what happens in Bottle 2, when soap is added?
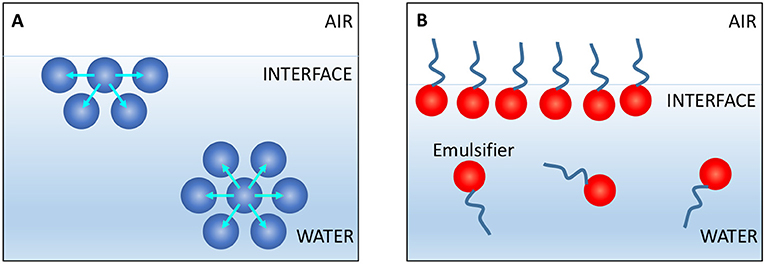
- Figure 3 - (A) Water molecules (blue spheres) like to be surrounded by other water molecules, but those at the air-water interface are partly surrounded by air.
- Thus, water molecules tend to minimize the amount of interface, even after being shaken. (B) Emulsifiers have hydrophilic heads (red spheres) and hydrophobic tails (blue lines), so part of the emulsifier wants to be in water but the other part wants to be in the non-water phase—the interface. This reduces the contact between the water molecules and the non-water phase, stabilizing the interface. Note: Emulsifiers are more than 10 times larger than water molecules.
Emulsifiers Save the Day Interface
Soap is filled with molecules called emulsifiers. Emulsifiers are very special molecules because they have a hydrophilic (water-loving) head and a hydrophobic (water-hating) tail (Figure 3B). So, one part of the emulsifier molecule wants to be in the water, while the other part prefers to be in the non-water phase (such as air or oil). There is actually a perfect place for these molecules to go to. Can you guess it? It is the interface!
As you know, one half of the interface is water, and the other half is air or oil. So, emulsifiers like to sit at the interface. Now, think about the shaking of Bottle 2. During shaking, we are pushing air into the water phase. When we do this, we create air bubbles with their own interfaces. The emulsifiers will go to the newly created interfaces. As a result, the water molecules are not in direct contact with the air anymore. Remember that water molecules do not want to be at the interface. The emulsifiers in soap reduce the contact between the air and water molecules at the interface. If you keep shaking, more new interfaces are formed, creating many air bubbles, and finally a layer of foam. This is what we call stabilization—the emulsifiers stabilize the interface, which makes the air bubbles and resulting foam last longer.
The two-bottle experiment also works for water and oil. The hydrophobic part of emulsifiers molecules also likes to be in oil. By shaking a bottle containing oil, water, and soap, you create many small oil droplets. The size of the oil droplets is determined by the energy with which the bottle is shaken—if you shake longer and more vigorously, you will get smaller oil droplets. This is actually what happens in food factories during the production of your favorite ice cream and mayonnaise.
The Search for Sustainable Emulsifiers
The emulsifiers in soap work very well, but they have disadvantages. They must be created using intense chemical processes and they are not edible, so they cannot be used in the production of foods like ice cream and mayonnaise. However, there are edible emulsifiers, such as proteins. Proteins are present in milk, eggs, and many other edible products. Therefore, milk and eggs are often used to create foods with lots of interfaces. For instance, milk is used to create milkshakes and ice creams. Eggs are used to create mayonnaises. Nowadays, we want emulsifiers that are sustainable, meaning those that are not harmful for the environment [3]. Sustainable emulsifiers can be found in plants like pea, soy, or wheat [4]. Plant-based emulsifiers contain proteins, fibers, and fats that can all stabilize interfaces. Plant-based emulsifiers are already used in many products, such as ice creams, cappuccinos, and plant-based dairy alternatives. These more sustainable, plant-based emulsifiers are also being used in other products, such as body lotions and detergents.
In summary, fluid interfaces are unstable by nature but can be easily stabilized by emulsifiers. Many emulsifiers are available to enlighten your life, in countless daily-life applications. But we are not there yet, as we need to keep searching for new, better and more sustainable emulsifiers. Are you going to help us?
Glossary
Fluid: ↑ A liquid, gas, or other material that can flows when a force is applied.
Immiscible: ↑ Describes two liquids that cannot be mixed, like oil and water.
Interface: ↑ A boundary where two immiscible fluids meet.
Emulsion: ↑ Mixture with two or more liquids that are immiscible, where one liquid exists as droplets in the other liquid, for instance oil droplets in water.
Foam: ↑ Mixture with a gas and a liquid, where many gas bubbles are surrounded by the liquid.
Emulsifier: ↑ A molecule that is partly hydrophilic and partly hydrophobic.
Hydrophilic: ↑ Describes something that likes to be in water/is attracted to water.
Hydrophobic: ↑ Describes something that does not like to be in water/repels water.
Stabilization: ↑ To make something stable, in this case, using emulsifiers to make an interface stable. An interface without emulsifier would collapse.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
[1] ↑ Murray, B. S. 2020. Recent developments in food foams. Curr. Opin. Colloid Interface Sci. 50:101394. doi: 10.1016/j.cocis.2020.101394
[2] ↑ Rühs, P. A., Bergfreund, J., Bertsch, P., Gstöhl, S. J., and Fischer, P. 2021. Complex fluids in animal survival strategies. Soft Matter 17:3022–36. doi: 10.1039/d1sm00142f
[3] ↑ Hinderink, E. B. A., Boire, A., Renard, D., Riaublanc, A., Sagis, L. M. C., Schroën, K., et al. (2021). Combining plant and dairy proteins in food colloid design. Curr. Opin. Colloid Interface Sci. 56:101507. doi: 10.1016/j.cocis.2021.101507
[4] ↑ Yang, J., and Sagis, L. M. C. 2021. Interfacial behavior of plant proteins–novel sources and extraction methods. Curr. Opin. Colloid Interface Sci. 56:101499. doi: 10.1016/j.cocis.2021.101499
