Abstract
Fluorine is the 13th-most abundant element on earth, found most often bound to other elements in its negatively charged form, fluoride. Fluoride compounds are used to improve dental health, to make steel, and to make useful materials like Teflon. Fluoride is also emitted into the environment as a byproduct of both natural and industrial processes. Fluoride even contaminates the fertilizer used to help plants grow. In high amounts, fluoride can be toxic. Single-celled organisms like bacteria protect themselves by making a transporter that specifically removes fluoride from the cell. Yeast have a similar transporter called FEX (f luoride exporter). Bacteria and yeast without these transporters die in the presence of the small amount of fluoride found in tap water. Plants are more complicated, but they also use FEX to keep fluoride from building up inside themselves. Plants without FEX can not make new seeds when grown in normal soil.
Fluoride: Not Just in Toothpaste
All living things require water to live. In the natural environment, water is not pure, but contains small amounts of other chemicals, many of which are beneficial to life. Organisms require a small amount of these chemicals to survive, but not too much. The amount needed is different for each chemical and each organism. There are also chemicals in the environment that are not required for life, and which can be harmful at certain levels. Fluoride is one of these chemicals.
Fluorine is the 13th-most abundant element on earth, found most often bound to other elements in its negatively charged form, the fluoride ion (F-). Fluoride is naturally released into water and air by erosion of fluoride-containing minerals, and from volcanoes and oceans. Additional fluoride is released into the environment by human activities like burning coal, making materials like steel and Teflon, and using fertilizers, which are made from rocks that contain fluoride. Fluoride is found everywhere on Earth in varying amounts.
Although fluoride is not required by living things, it can be beneficial in low doses. The best example of this is the strengthening of tooth enamel to prevent cavities. However, fluoride is toxic in high amounts. Because it is found everywhere, bacteria, plants, and animals all must be able to survive in environments that contain fluoride.
Why Is Too Much Fluoride Bad?
Fluorine is at the top right corner of the periodic table of elements. That is where the most electronegative elements are found, which means that fluorine is extremely likely to grab an extra electron and become F-. This property makes fluoride really good at bonding with other things. For example, it can combine with calcium and phosphate to form very strong tooth enamel that is resistant to damage from bacteria, preventing cavities. But too much fluoride can have negative effects. By bonding with calcium too well, fluoride can interfere with healthy bone formations. Fluoride can also stick to proteins and keep them from functioning properly. Toxicity from too much fluoride can happen to microorganisms, plants, and even people.
Organisms Protect Themselves From Fluoride
For fluoride to damage a cell, it must first get inside (Figure 1). Each cell is surrounded by a membrane that separates the inside from the outside. The membrane keeps out things that are too large or that have a positive or negative charge. Since the fluoride ion has a negative charge, it can not cross the membrane on its own (Figure 1A). However, fluoride can bind to a positively charged hydrogen atom (H+), to make a new molecule called hydrogen fluoride, which has no charge and is small enough to easily pass through the membrane (Figure 1B). Once inside the cell, hydrogen fluoride can fall back apart into H+ and F-. Since F- is charged, it can not get back out of the cell, so it builds up and can harm the biomolecules inside (Figure 1C).
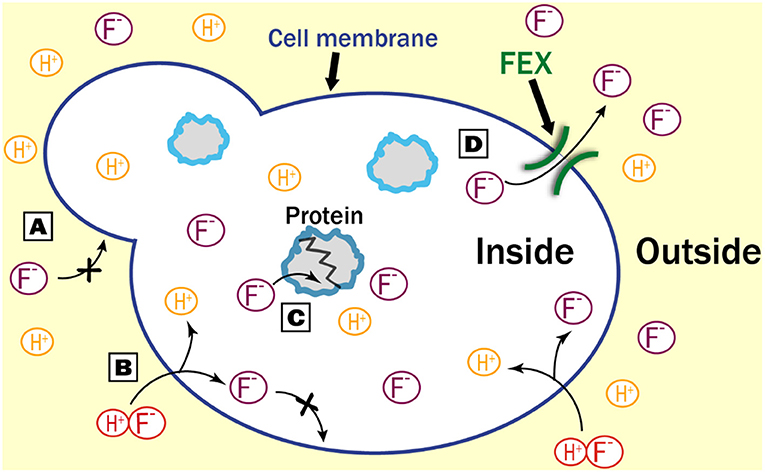
- Figure 1 - How does fluoride get in and out of cells?
- (A) In the absence of FEX, negatively charged fluoride ions (F-) cannot cross the cell membrane. (B) However, if F- bonds with a positively charged hydrogen (H+) to form hydrogen fluoride, it can enter the cell easily. Once inside, F- breaks apart from H+ and becomes trapped. (C) In the cell, F- can stick to proteins and prevent them from working. (D) FEX makes an opening in the cell membrane, through which only F- can pass to exit the cell.
How do living things get this toxic ion back out of cells? In single-celled organisms like bacteria and yeast, there is a transporter in the cell membrane that specifically allows F- to leave the cell (Figure 1D) [1–3]. This keeps the amount of fluoride in the cell at low, nontoxic levels. One version of this protein is called fluoride exporter (FEX). When scientists removed FEX from bacteria or yeast cells, those cells could no longer grow—even in environments with small amounts of fluoride. This means that FEX is the main way that single-celled organisms keep the amount of fluoride inside their cells at a nontoxic level.
Plants Are More Than a Collection of Cells
Life gets more complicated when many cell types must work together to form an organism like a plant. Plants move a tremendous amount of water in the transpiration stream from their roots to the tips of the leaves and, when fluoride is dissolved in the water, it also moves throughout the plant (Figure 2). You can see the transpiration stream for yourself by putting a celery stalk in a glass of water with red food coloring. Over time, the celery leaves will turn red as the water and food coloring are pulled up. If you cut across the stalk, you can see red dots, which are the xylem—the tubes that carry water from the roots.
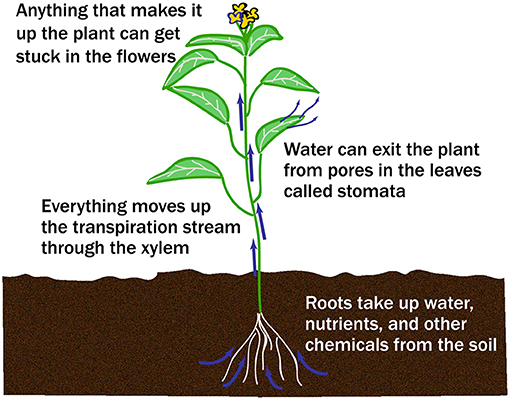
- Figure 2 - In a plant, water moves from the roots to the leaves and flowers through the transpiration stream, traveling through a tube-like plant tissue called xylem.
- Nutrients and other materials, like fluoride, are carried along with the water.
To help plants grow, nutrient-containing fertilizers are added to soil. Many fertilizers are made from rocks that contain fluoride. This means plants can get fluoride from soil, water, fertilizer, and even from the air. For an organism with multiple cells working together, getting fluoride out of a single cell might not be good enough if the fluoride gets stuck nearby and kills a different cell. One way to keep the plant safe would be if some cells worked together to keep fluoride out of the plant entirely. Alternatively, each individual cell could push fluoride out into the transpiration stream like a game of “hot potato” until fluoride makes it all the way to the tips of the plant and exits along with water. Because of this added complexity, we wanted to see whether plants used the same method to protect themselves from fluoride as yeast.
We found that all plants also have the FEX protein. When we took the natural FEX out of a yeast cell, that cell died in just a little bit of fluoride, but if we replaced it with a plant FEX protein, the yeast cell survived [4]. This worked with FEX from any plant we tried, including moss, lettuce, tea, and Arabidopsis—a plant commonly used in lab experiments [5]. This means that plant FEX can work the same way as yeast FEX, to move fluoride out of a cell.
To show that FEX was also essential for fluoride tolerance in plants, we made a mutation—a change in the plant’s instructions for making FEX—that inactivated FEX within the plant. We did this using the common laboratory plant Arabidopsis [5]. If the mutant plants were grown in water with no soil so that no fluoride was present, they looked just like normal plants. If they were grown in soil with just a tiny bit of fluoride, they were okay until they started to flower—then their flower stalks were shorter and the flowers started dying (Figures 3A, B). The plants without FEX never made seeds, even when the flowers did not die. Close examination showed that, in low fluoride, the plants’ pollen was defective, which explains why they could not produce seeds.
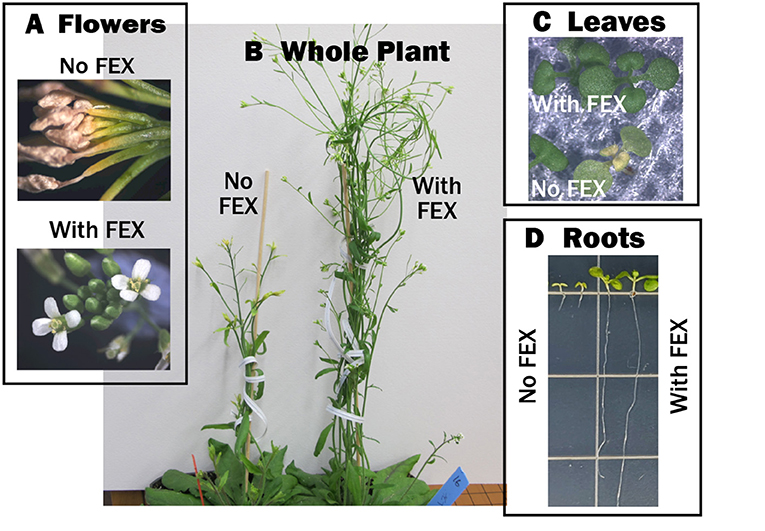
- Figure 3 - FEX keeps plants healthy when they are exposed to fluoride.
- When FEX is not functioning, fluoride causes: (A) the flowers to wilt and die, (B) the flower stems to grow shorter, (C) yellow leaves, and (D) the roots to be much shorter.
When fluoride was added to mutant seedlings (baby plants), the leaves became yellow and unhealthy (Figure 3C). When the plants were grown in a special gel so we could see the roots, adding just a little bit of fluoride caused the roots to be extremely short (Figure 3D). When FEX was added back to the mutant plant, it could grow again, even in relatively high amounts of fluoride. In fact, if plants made a higher than normal amount of FEX, they grew even better in fluoride than normal plants. All these experiments show that FEX is responsible for keeping plant cells alive when there is fluoride in the environment.
What Does This Mean for Us?
We have known for many years that some plants are more sensitive to fluoride than others. We rely on some fluoride-sensitive plants, like corn and peaches, for food. We did not know why some plants were less affected by fluoride in the environment, but now we have learned that FEX can protect plants. Now that we have discovered the main mechanism used by plant cells to keep fluoride from building to toxic levels, we can manipulate both FEX itself and how and where it is made in the plant. Our hope is that, by tweaking FEX, we can make some crop plants grow better—despite the presence of fluoride in the environment and in fertilizer.
Animals, like plants, must deal with the toxic effects of fluoride build up, so we expect that there is something like FEX in other animals, including humans. So far, scientists have not identified how fluoride is removed from animals—the most complex animal found to have FEX is a sea sponge. There are many animal proteins with unknown functions, and some of them are known to cause diseases when they are not functioning properly. It is possible that one of these proteins is like FEX, and that animals also experience a disease related to fluoride. Scientists will only know this for certain once they figure out, through future research, how humans and other animals protect themselves from the dangerous effects of fluoride.
Glossary
Fluoride (F-): ↑ The negatively charged form of the element fluorine; the form found most often in the environment.
Ion: ↑ The charged version of an atom or molecule, formed by gaining or losing negatively charged electrons.
Electronegative: ↑ Quality of an atom that causes it to attract electrons so that it becomes a negative ion.
Membrane: ↑ The barrier surrounding an individual cell, which keeps most material from passing through it.
Transporter: ↑ A protein located in the cell membrane that makes an opening that specific molecules can pass through.
Fluoride Exporter (FEX): ↑ A membrane transporter that is specific for the fluoride ion.
Transpiration Stream: ↑ The path that water takes up a plant, from the roots to the leaves.
Xylem: ↑ The plant tissue that acts as a tube, carrying water and nutrients from the roots.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Tanya Berbasova and Martin Peverelli, the other authors of the original research paper. We also thank all the other members of the Strobel lab for their discussions and ideas. We also acknowledge support from the National Science Foundation (Grant number GR108639).
Original Source Article
↑Tausta, S. L., Berbasova, T., Peverelli, M., and Strobel. S. A. 2021. The fluoride transporter FLUORIDE EXPORTER (FEX) is the major mechanism of tolerance to fluoride toxicity in plants1. Plant Physiol. 186:1143–58. doi: 10.1093/plphys/kiab131
References
[1] ↑ Baker, J. L., Sudarsan, N., Weinberg, Z., Roth, A., Stockbridge, R. B., and Breaker, R. R. 2012. Widespread genetic switches and toxicity resistance proteins for fluoride. Science 335:233–5. doi: 10.1126/science.1215063
[2] ↑ Li, S., Smith, K. D., Davis, J. H., Gordon, P. B., Breaker, R. R., and Strobel, S. A. 2013. Eukaryotic resistance to fluoride toxicity mediated by a widespread family of fluoride export proteins. Proc. Natl. Acad. Sci. U.S.A. 110:19018–23. doi: 10.1073/pnas.1310439110
[3] ↑ Stockbridge, R. B., Lim, H. -H., Otten, R., Williams, C., Shane, T., Weinberg, Z., et al. 2012. Fluoride resistance and transport by riboswitch-controlled CLC antiporters. Proc. Natl. Acad. Sci. U.S.A. 109:15289–94. doi: 10.1073/pnas.1210896109
[4] ↑ Berbasova, T., Nallur, S., Sells, T., Smith, K. D., Gordon, P. B., Tausta, S. L., et al. 2017. Fluoride export (FEX) proteins from fungi, plants and animals are ’single barreled’ channels containing one functional and one vestigial ion pore. PLoS ONE 12:e0177096. doi: 10.1371/journal.pone.0177096
[5] ↑ Tausta, S. L., Berbasova, T., Peverelli, M., and Strobel. S. A. 2021. The fluoride transporter FLUORIDE EXPORTER (FEX) is the major mechanism of tolerance to fluoride toxicity in plants1. Plant Physiol. 186:1143–58. doi: 10.1093/plphys/kiab131
