Abstract
Nitrogen is essential to all life on Earth. Tiny organisms, called microbes, help to make nitrogen available to all other living things. One group of microbes, called nitrite-oxidizing bacteria (NOB), help to remove excess nitrogen from the environment that can be dangerous to plants and animals. However, there is still a lot to learn about the distribution and function of NOB in nature. In the lab, we grew new types of NOB from rivers. By studying their DNA, we found that these NOB can gain energy in many ways and survive the stress of pollution. Similar NOB seem to be present all over Earth. Studying NOB can help us to understand how changes to the environment might affect microbes and their habitats.
Microbes are Essential for Nitrogen Cycling
Nitrogen is important for all life, including microbes, plants, and animals, because it is used to build cell structures like DNA and proteins. However, most nitrogen occurs as a gas in the air that is not usable by plants and animals. Only certain types of microbes can convert unusable nitrogen gas into forms that plants and animals can use. There are several types of microbes that can consume and produce various usable forms of nitrogen. Other types of microbes turn these usable forms of nitrogen back into nitrogen gas, which keeps nitrogen levels balanced in the ecosystem. This cycle, called the nitrogen cycle, is continuous (for more information about the nitrogen cycle, see this Frontiers for Young Minds article). Microbes play a huge role in the nitrogen cycle—without them, there would not be enough usable nitrogen for plants and animals. Microbes can also help balance nitrogen levels when human activities add too much nitrogen to the environment, such as through the use of nitrogen fertilizers. Thus, these microbes are essential for supporting life and maintaining healthy ecosystems.
Why are Nitrite-Oxidizing Bacteria Important?
Nitrite-oxidizing bacteria (NOB) are an important group of microbes involved in the nitrogen cycle. NOB consume a form of nitrogen called nitrite () and turn it into another form called nitrate (). Excessive buildup of nitrites can be toxic and can prevent certain organisms from using oxygen appropriately. Production of nitrate is important because this is the form of nitrogen that certain plants use to grow. The nitrate produced by NOB is also consumed by other microbes that can help to remove excess nitrogen from the ecosystem. So, NOB are important for keeping the nitrogen cycle running. If NOB activity decreases or stops due to stressful conditions such as pollution or temperature changes, then the whole nitrogen cycle would ultimately become unbalanced (Figure 1). This would lead to a slow breakdown of the environment, and plants and animals would suffer as a result.
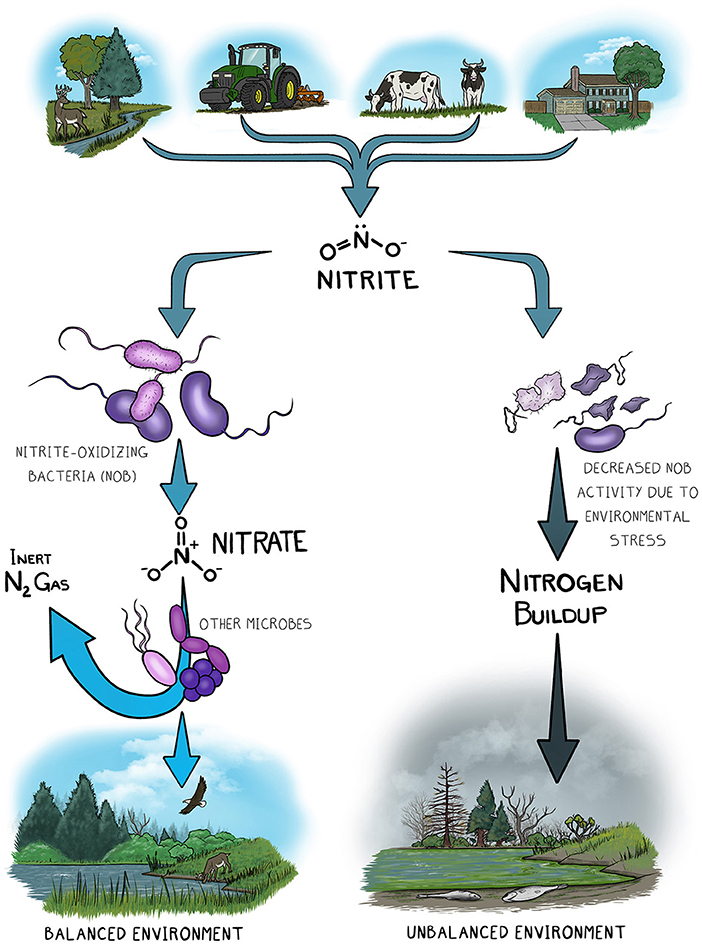
- Figure 1
- Nitrogen enters the environment in various forms including nitrite from natural sources (e.g., certain types of bacteria) and unnatural sources (e.g., pollution). If NOB are present and healthy, they convert nitrite to nitrate, a form of nitrogen that can be used by other organisms that help to remove nitrogen. This keeps the environment in balance. However, if NOB activity is decreased due to stresses like pollution or temperature, nitrogen builds up and throws the environment out of balance.
A New Type of NOB in Colorado Rivers
Learning about various types of NOB is useful since each type of NOB has unique functions and can survive in unique conditions. So, studying these microbes helps us to understand the important contributions they are making in various environments. We were interested in learning which types of NOB live in Colorado rivers and how they might help to keep the rivers healthy. Prior studies showed that two types of NOB, Nitrobacter and Nitrospira, are commonly found in rivers around the world [1, 2], so we wanted to see if they were also found in Colorado rivers.
We collected river water and sediments, brought them back to the lab, and grew them in cultures with the nutrients and conditions (such as temperature) that NOB need [3]. Instead of Nitrobacter and Nitrospira, we found four new species of NOB in our river cultures, called Candidatus Nitrotoga. The prefix “Candidatus” (abbreviated Ca.) is temporary until these NOB are formally named and approved by a certain group of scientists. Nitrotoga is the name of the genus, which is a category of organisms that have common characteristics based on their physical features or DNA. The four species that we found (named for the areas that they were collected from) have differences from each other that make them unique.
We were surprised to find these new NOB in our cultures because Ca. Nitrotoga had previously been found mostly in wastewater, sediments, and glacial soils [1, 2]—these organisms had never been cultured from natural freshwater environments. Since only a few types of Ca. Nitrotoga had been studied in detail, there was still a lot to learn about them.
Learning More About Ca. Nitrotoga
We started to learn about Ca. Nitrotoga by looking at it under a microscope to reveal the sizes and shapes of the cells, and by growing it in the lab to determine which chemical and physical conditions it prefers. Then we sequenced Ca. Nitrotoga genomes to help us identify thousands of additional cell features, which opened the mysterious world inside the microbes in a way that could not be revealed with other laboratory tools (for more information on genome sequencing, see this Frontiers for Young Minds article). Ca. Nitrotoga gene sequences were compared to other bacterial genes with known functions to look for matches. Genetic matches were used to predict where Ca. Nitrotoga might live and what roles they might play in the environment. Gene sequences provided information about cell structures, metabolism, and how these microbes respond to stresses like pollution or climate change (Figure 2A).
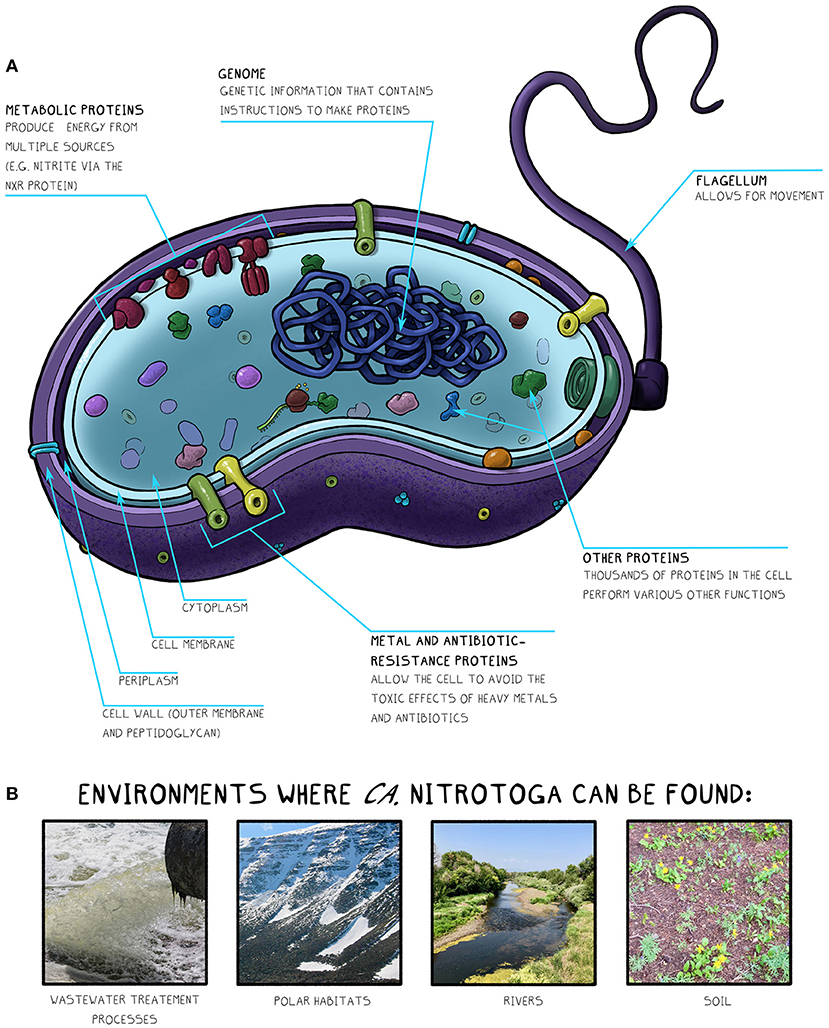
- Figure 2
- (A) Diagram of a Ca. Nitrotoga cell showing key features identified by genome sequencing. (B) Ca. Nitrotoga has been found in various habitats, including in wastewater, polar regions, rivers, and soil.
What Does Ca. Nitrotoga “Eat” and “Breathe”?
Imagine only ever eating one type of food, like cookies, all day every day! NOB, including Ca. Nitrotoga, were once thought to use only nitrite to get the energy needed for their growth, movement, and other functions. However, Ca. Nitrotoga (and other NOB) genomes contain genes that suggest these microbes may also use other energy sources, such as sulfur, hydrogen, and organic carbon [3]. This is like being able to “eat” more types of food. We know that Ca. Nitrotoga can “breathe” oxygen to grow, but the genomes showed that they may also survive in low-oxygen conditions [3]. Overall, these findings suggest that Ca. Nitrotoga can “eat” and “breathe” a variety of compounds. Ca. Nitrotoga bacteria impact the Earth differently depending on which compounds they “eat” and “breathe.” These microbes are likely to live in many different habitats because of this flexibility, and it is likely that NOB can keep growing even when their food sources change.
Can Ca. Nitrotoga Survive in Contaminated Environments?
The Colorado NOB live in rivers contaminated with metals (like gold, copper, and arsenic) and antibiotics (chemicals used to kill bacterial infections but that can also harm helpful bacteria). These contaminants enter the river through wastewater and as runoff from industries and farms. Metals and antibiotics can be toxic to microbes, but the Ca. Nitrotoga genomes suggest that these microbes have defense strategies to protect them from these harmful substances [3]. For example, Ca. Nitrotoga may be able to pump metals and antibiotics back outside the cell, before they can cause too much damage. Ca. Nitrotoga may also be able to convert metals and antibiotics into less-toxic forms by changing the chemical structures. We tested this in the lab and showed that the river Ca. Nitrotoga species continued to consume nitrite in the presence of several antibiotics [4]. These strategies likely help Ca. Nitrotoga survive in habitats polluted with toxic metals and antibiotics, meaning they can probably continue to cycle nitrogen and keep the ecosystem balanced even in contaminated conditions.
Where Else Do Ca. Nitrotoga Live?
Using databases containing gene sequences from many microbes, we found sequences similar to Ca. Nitrotoga on all continents and in many habitats, including freshwater, saltwater, sediment, soil, and wastewater treatment plants (WWTP) (Figure 2B) [3]. Ca. Nitrotoga-like sequences were found in habitats with temperatures ranging from 0–33°C. Overall, this analysis extended the range of where scientists think Ca. Nitrotoga can live. Their flexible metabolisms and adaptation strategies likely help these NOB survive in diverse environments around the world.
Are River Ca. Nitrotoga Genomes Unique?
We compared the Colorado river Ca. Nitrotoga genomes to the recently published genomes of other Ca. Nitrotoga species found in a WWTP in Austria [5] and from coastal sediments in Japan [6]. All these Ca. Nitrotoga species were predicted to turn nitrite into nitrate, as expected. Interestingly, the protein that all of these Ca. Nitrotoga species used to do this appeared to be slightly different from other types of NOB, which may impact how fast they can consume nitrite. The Ca. Nitrotoga species also shared other traits with known NOB species, such as using various ways to gain energy. Some Ca. Nitrotoga genomes had genes used to build a flagellum (a tail-like structure that helps bacteria move) while others did not. Many Ca. Nitrotoga genomes contained protection mechanisms for survival in stressful environments, but each species also had unique protection mechanisms to handle specific stresses. Exploring similarities among the Ca. Nitrotoga genomes helps scientists understand how NOB grow and thrive, whereas exploring the differences highlights how individual species adapt to specific habitats.
So, Now You Know!
In summary, NOB are important because they help maintain healthy ecosystems. Our study found new types of NOB in Colorado rivers, grew them in the lab, and sequenced their genomes. Genome sequence data helped us better understand how these bacteria might function in the environment and survive under stressful conditions. For instance, they can still cycle nitrogen even in the presence of antibiotic pollution. Comparisons with other closely related bacteria showed some similarities and some differences. Overall, our work highlights why scientists need to keep studying NOB to learn about how these microbes function in various habitats across the Earth.
Glossary
Ecosystem: ↑ The organisms and physical environment in a particular area (such as a river or forest).
Nitrogen Cycle: ↑ How nitrogen changes into different forms (such as nitrite and nitrate) and moves through different parts of the Earth including living organisms, land, oceans, and air.
Nitrite-Oxidizing Bacteria (NOB): ↑ Bacteria that turn nitrite into nitrate as part of the nitrogen cycle.
Sequencing: ↑ The process of determining the order of the DNA bases in genes and genomes.
Genome: ↑ The complete set of genetic information within a cell (DNA for most organisms) containing all of the necessary information to function.
Gene: ↑ Short stretches of DNA within the genome that contain instructions to make proteins.
Metabolism: ↑ Chemical processes in a cell that maintain life, such as building cell structures and producing energy.
Wastewater: ↑ Water than has been previously used in different ways, such as water running off fields on farms or water entering a river after sewage treatment.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Original Source Article
↑Boddicker, A. M., and Mosier, A. C. 2018. Genomic profiling of four cultivated Candidatus Nitrotoga spp. predicts broad metabolic potential and environmental distribution. ISME J. 12:2864–82. doi: 10.1038/s41396-018-0240-8
References
[1] ↑ Ward, B.B., Arp, D.J., and Klotz, M.G. 2011. Nitrification. Washington, DC: American Society for Microbiology Press.
[2] ↑ Daims, H., Lücker, S., and Wagner, M. 2016. A new perspective on microbes formerly known as nitrite-oxidizing bacteria. Trends Microbiol. 24:699–712. doi: 10.1016/j.tim.2016.05.004
[3] ↑ Boddicker, A.M., and Mosier, A.C. 2018. Genomic profiling of four cultivated Candidatus Nitrotoga spp. predicts broad metabolic potential and environmental distribution. ISME J. 12:2864–82. doi: 10.1038/s41396-018-0240-8
[4] ↑ Lantz, M.A., Boddicker, A.M., Kain, M.P., Berg, O.M.C., Wham, C.D., and Mosier, A.C. 2021. Physiology of the nitrite-oxidizing bacterium Candidatus Nitrotoga sp. CP45 enriched from a Colorado river. Front. Microbiol. 12:709371. doi: 10.3389/fmicb.2021.709371
[5] ↑ Kitzinger, K., Koch, H., Lücker, S., Sedlacek, C.J., Herbold, C., Schwarz, J., et al. 2018. Characterization of the first “Candidatus Nitrotoga” isolate reveals metabolic versatility and separate evolution of widespread nitrite-oxidizing bacteria. mBio. 9:e01186-18. doi: 10.1128/mBio.01186-18
[6] ↑ Ishii, K., Fujitani, H., Sekiguchi, Y., and Tsuneda, S. 2020. Physiological and genomic characterization of a new ‘Candidatus Nitrotoga’ isolate. Environ. Microbiol. 22:2365–82. doi: 10.1111/1462-2920.15015
