Abstract
Water is essential for life. The particles and dissolved chemicals found in rivers, lakes, and oceans are constantly changing with weather, seasons, and human activities. The substances found in water can be helpful or harmful to humans and other organisms. New technologies allow scientists to use waterproof computers (called sensors) to record the quality of water as it changes throughout the day or night. Many sensors use the interaction of water with light, or other energy forms, to learn about what is in it. Through observing what happens to light energy in water, scientists can reveal the sources and movements of harmful pollutants or essential nutrients for plants, animals, and bacteria. Networks containing many sensors work together to provide continuous information about the ever-changing conditions that affect all living organisms that depend on water.
What is in the Water?
Everyone relies on water to survive, but not just any water will do. Humans cannot safely drink the salty water in the oceans, and we are not usually tempted to drink out of muddy puddles in a parking lot. To safely use water, we need to know what is in it: the water quality. Answering the question, “What is in the water?” is both an exciting and challenging task, and new technologies can provide us with better answers. Liquid water is constantly on the move, and it carries both healthy and harmful substances from one place to another. Measuring pollutants and nutrients to decide what levels are safe or harmful presents a challenge that society faces each day.
Scientists typically analyze water by collecting samples—small amounts of water from the sources they are interested in testing. Some solids we can normally see seem to disappear once they are dissolved in water. Sugar and salt are good examples. Larger solids such as dirt, insects, and trash are easier to spot as they get swept up by flowing water. Scientists call these larger items “particles” because they are too large to pass through most water filters. Learning how much and what types of dissolved chemicals and particles are present in water samples requires specialized equipment. Water scientists, called hydrologists, act like laboratory detectives, by using instruments to tease apart the mysteries in water, step by step.
However, there is a problem with the traditional approach of collecting water samples. Before a sample makes it to the laboratory, the quality of water in the rivers, lakes, and oceans may have already changed. The constantly changing water quality in the environment may replace an old mystery with a new one, within a matter of minutes. That is why hydrologists and other types of scientists who study water take measurements repeatedly each year, month, week, or even every day (Figure 1). In fact, these scientists are always looking for new ways to learn about how water quality is changing from minute to minute as well as from decade to decade!

- Figure 1 - Collecting water samples takes time and effort.
- (A) A scientist fills a bottle with a water sample. (B) A scientist analyzes water chemistry in a laboratory.
New Tools for An Old Problem
Thankfully, some technologies give scientists a helping hand. Waterproof computers known as sensors attached to docks, bridges, and buoys can record measurements even when no one is nearby, and even in the middle of the night! These water-quality sensors are programmed to wake up, interact with the water, and take notes before going back to sleep. As often as every few seconds, sensors can take the physical and chemical “pulse” of the water [1]. Some sensors have been used for decades to monitor and protect water quality and habitats for fish. Water-treatment facilities also use sensors to prepare water for drinking, or for making sure our waste waters are clean enough to be returned to the environment.
Over time, technological advances have generated new types of sensors that help us uncover new mysteries. These include sensors that use the characteristics of light and other forms of energy to detect some important molecules and particles that are present in rivers, lakes, and oceans [2]. The new sensors use the interaction of certain forms of chemical elements, such as carbon and nitrogen, with light under water. Other substances that interact with light include suspended particles such as soil (earth) and even water itself. One advantage of using light to measure water quality is that it does not change the water during measurement. Using sensors to collect data allows water to stay where it is without taking a lengthy and costly detour to the laboratory!
How to Measure Light Under Water
Different types of light contain different amounts of energy. These differences, for example, are what create all the colors we see. Water gets in the way of red light more than blue light, blocking red light from reaching our eyes. That is why water often appears blue to the human eye. Ultraviolet light is more energetic than blue light, and blue light is more energetic than red light. The different energies of light are analogous to different sounds. Sound that is so high that dogs can hear it but humans cannot is like ultraviolet light, which is too high energy for humans to see. Light travels through space, air, and water until an obstacle gets in the way. Measuring the obstacles in light’s path underwater can tell us a lot about water quality.
Measuring water quality with light requires a light source positioned across from a light detector (Figure 2). Water flows through the space between the source and the detector. The light emitted from the light source passes through the water while interacting with all the molecules and particles in the water. These interactions are like a person bumping shoulders while moving through a crowded room, pausing for an occasional handshake. Comparing the amount of light that reaches the detector to the light that leaves the source generates interesting data that hydrologists can use. The greater the distance between the light source and the detector, the more opportunity light has to interact with substances along its path. The loss of light reaching the detector can tell scientists about what is in the water.
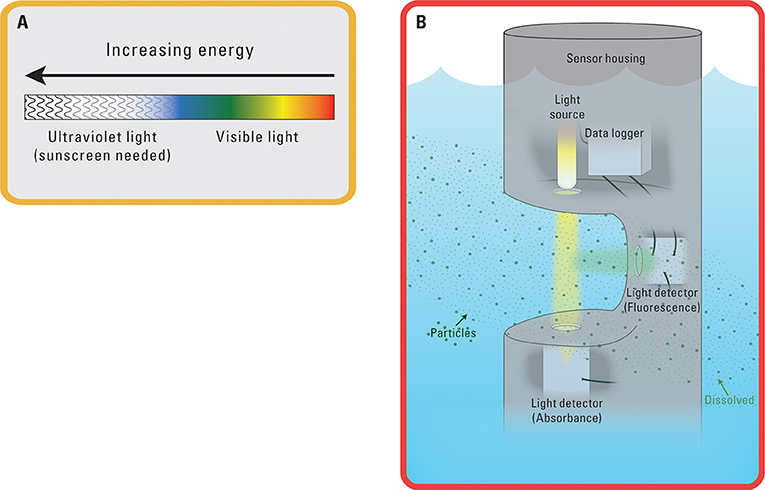
- Figure 2 - Simple on the outside, complex on the inside! (A) Visible and ultraviolet light are forms of energy.
- (B) Sensors can take a variety of forms. Many are cylinders protected by a plastic or metal covering. Hiding inside are the energy sources, detectors, and computers for logging (storing) data.
Scientists must consider many possibilities when interpreting the results from underwater light sensors. Larger particles can physically block light’s path the way a person casts a shadow on the ground in the late afternoon sun. Some light is also stopped in its path when its energy is transferred to certain dissolved molecules. When light is absorbed by a molecule, the light’s energy can transform into movement and vibrations within that molecule. A molecule might “feel” light during absorbance similar to the way people feel the sun’s heat on a sunny day. Another possibility is that the light energy absorbed by a molecule is transformed into fluorescence. Fluorescence occurs when light energy enters a molecule as one form and leaves the molecule as a different form. This newly created fluorescent light can also be measured with an underwater light detector. Hydrologists know that only certain types of chemicals can emit fluorescent light. All these interactions decrease the amount of light that passes from the light source to the light detector within the sensor.
When the Results Come In
What kinds of mysteries are solved using waterproof sensors, light sources, and light detectors? Scientists are using sensors to solve mysteries about the continuous movements of two essential elements for life: nitrogen and carbon. Nitrate is a common form of nitrogen dissolved in water that plants and bacteria eat. When plants, animals, and bacteria decompose, the dissolved molecules containing carbon and other elements are called dissolved organic matter. Dissolved organic matter washes into nearby rivers, lakes, and oceans. When dissolved in water, nitrate and organic matter interact with ultraviolet and visible light through absorbance, fluorescence, or both. Monitoring both substances improves our understanding of ecosystems and helps determine whether the water is safe for humans to use.
For example, sensors improve scientists’ calculations of the amount of these substances flowing down a river by recording measurements, even during dangerous floods. The frequent reports recorded by sensors give scientists an understanding of how these substances change as water flow increases. The resulting data can indicate whether nitrate came from natural forests, human wastewater (sewage), or farms [3]. The data can also indicate when drinking water must be treated to reduce dissolved organic matter to a safe level [4]. As data are collected over many years, stories about the effects of seasonal weather and changing climate on water quality are beginning to unfold. Longer droughts and more intense storms are changing river water quality over time.
Underwater Flashlights of The Future
With so many sensors working day and night, the amount of data adds up quickly. Even scientists that work in large teams need special tools to keep up with the large amount of data. Sensors report results back to scientists using cell phone towers and satellites (Figure 3). Data are stored across many computers within data centers all over the world. Data are carefully checked for errors before being used to make decisions. These elaborate connections between multiple sensors and computers are called water-quality sensor networks. Sensor networks have already contributed to many discoveries that were not previously possible, and even more sensors are waiting to record water’s stories.
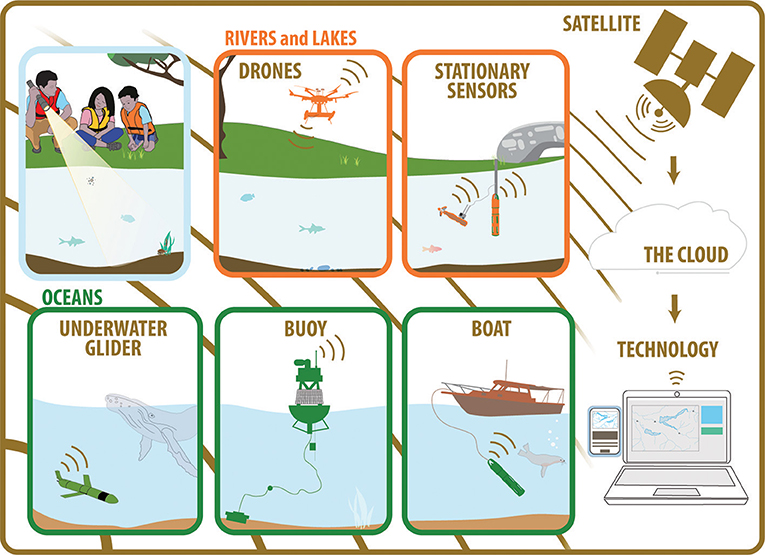
- Figure 3 - The many connections within water-quality networks.
- Stationary (unmoving) and mobile (moving) sensors report results continuously, by communicating with computers through satellites or cell phone towers. The data are processed to create graphs that allow scientists to monitor water quality.
New ways to use sensors to learn even more about rivers, lakes, and oceans are being investigated. For example, sensors attached to boats, submarines, and gliders are being used to map changing water quality over longer distances underwater. From the sky above, drones, airplanes, and satellites can observe even more interactions of light with water surfaces. Machine learning, a form of artificial intelligence, is now being applied to interpret sensor data to make more accurate predictions. The need to monitor and predict water quality in rivers, lakes, and oceans will only increase over time, as societies grow. The next generation of water detectives are sure to continue using new technologies to help solve the water mysteries of the future!
Glossary
Hydrologist: ↑ A scientist that studies the properties, distribution, and effects of water on the Earth’s surface, in the soil and underlying rocks, and in the atmosphere.
Sensor: ↑ A device that measures observable changes in matter or energy.
Ultraviolet Light: ↑ A form of light energy present in sunlight that contains more energy than visible light but less energy than X-rays.
Absorbance: ↑ A process by which light energy is transferred to matter or a molecule.
Fluorescence: ↑ A process by which a molecule emits energy in the form of light.
Nitrate: ↑ A molecule made of one nitrogen atom and three oxygen atoms and that is a nutrition source for living things commonly found in rivers, lakes, oceans, and wastewaters.
Dissolved Organic Matter: ↑ A complex mixture of carbon-based molecules dissolved in water, primarily from the decomposition of plants, bacteria, and algae. “Organic” here is different from organic food in the supermarket.
Machine Learning: ↑ A field of computer science and statistics that uses data inputs and algorithms to make predictions (outputs) and to find useful patterns in data.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Written informed consent was obtained from the individual(s) for the publication of any identifiable images or data included in this article.
Original Source Article
↑Burns, D. A., Pellerin, B. A., Miller, M. P., Capel, P. D., Tesoriero, A. J., Duncan, J. M. 2019. Monitoring the riverine pulse—Applying high-frequency nitrate data to advance integrative understanding of biogeochemical and hydrological processes. WIREs Water 6:e1348. doi: 10.1002/wat2.1348
References
[1] ↑ Rode, M., Wade, A. J., Cohen, M. J., Hensley, R. T., Bowes, M. J., Kirchner, J. W., et al. 2016. Sensors in the stream— The high-frequency wave of the present. Environ. Sci. Technol. 50:10297–307. doi: 10.1021/acs.est.6b02155
[2] ↑ Pellerin, B. A., Stauffer, B. A., Young, D. A., Sullivan, D. J., Bricker, S. B., Walbridge, M. R., et al. 2016. Emerging tools for continuous nutrient monitoring networks—Sensors advancing science and water resources protection. J. Am. Water Resour. Assoc. 52:993–1008. doi: 10.1111/1752-1688.12386
[3] ↑ Burns, D. A., Pellerin, B. A., Miller, M. P., Capel, P. D., Tesoriero, A. J., and Duncan, J. M. 2019. Monitoring the riverine pulse—Applying high-frequency nitrate data to advance integrative understanding of biogeochemical and hydrological processes. WIREs Water 6:e1348. doi: 10.1002/wat2.1348
[4] ↑ Ruhala, S. S., and Zarnetske, J. P. (2017). Using in-situ optical sensors to study dissolved organic carbon dynamics of streams and watersheds—A review. Sci. Total Environ. 575:713–23. doi: 10.1016/j.scitotenv.2016.09.113
