Abstract
We all know that carbon dioxide (CO2) is produced from burning fossil fuels, and that it contributes to global warming. But have you heard about the “evil twin” of global warming, also caused by CO2–ocean acidification? The ocean absorbs about 30% of the CO2 that humans emit each year. As CO2 dissolves into the ocean, it forms carbonic acid, effectively making the ocean acidic. Animals that use a substance called calcium carbonate to build their shells and skeletons are vulnerable to acidic conditions, as their hard parts may dissolve. Microscopic swimming snails called pteropods or sea butterflies are common in the Southern Ocean. With incredibly delicate shells thinner than a human hair, pteropods are often considered to be the organisms most vulnerable to ocean acidification. Using microscopes and X-rays, we took extremely detailed images of these tiny shells and found that pteropods have a couple of clever tactics to defend their “homes” against ocean acidification.
Ocean Acidification: The “other” CO2 Problem
As humans continue to burn fossil fuels, more and more carbon dioxide (CO2) builds up in the atmosphere. CO2 is a greenhouse gas, and for years we have understood that CO2 is responsible for global warming, which can make life difficult for some animal species. What we did not understand was that CO2 was also affecting the oceans and the animals that live there. We now know that the ocean absorbs 30% of the CO2 human activities produce each year. While CO2 absorbed by the oceans slows down global warming, it can be bad news for the oceans—and more so for ocean animals.
Animals such as mussels and crabs have shells made from calcium carbonate (CaCO3). These animals build calcium carbonate using carbonate ions (), which they find in the seawater they live in. The ease of building a shell depends on how many carbonate ions are around. When the concentration of carbonate ions is high, it is easy to build a shell.
When the oceans absorb CO2, the CO2 reacts with water to form carbonic acid, effectively making the ocean acidic. This process is called ocean acidification. In seawater, carbonic acid and carbonate ions exist in a kind of balance with each other. When CO2 is absorbed by the oceans and more carbonic acid is produced, the concentration of carbonate ions decreases. When the concentration of carbonate ions is low, the water is said to be undersaturated. Animals living in undersaturated water must work harder to collect the carbonate ions needed to build their shells. Also, undersaturated water can dissolve calcium carbonate, to “take back” the carbonate ions. As we burn more fossil fuels, more CO2 is absorbed by the oceans and undersaturated waters become increasingly common, threatening all ocean animals with shells.
Which Animals Are Most at Risk From Ocean Acidification?
Ocean acidification is most advanced in the oceans around the North and South Poles, where the concentration of carbonate ions is naturally low. Also, colder water absorbs more CO2 than warmer water does. Thus, the Arctic and Antarctic Oceans have the most undersaturated waters in the world, and the animals living there are most at risk. Sea butterflies are animals with calcium carbonate shells that live in the polar regions—so they fit the bill! A sea butterfly is a microscopic swimming snail (Figure 1). Despite their small size, there are so many of them in the Southern Ocean that they form an important part of the food chain. The Latin name for sea butterfly is pteropod, which means “winged foot.” What would be the foot of a “normal” snail has evolved into two wings in pteropods. The pteropod flaps its wings like a butterfly to swim through the ocean. The homes of these pteropods are incredibly delicate shells made from calcium carbonate. Pteropod shells are about 1,000 times thinner than 1 mm—even finer than a human hair. The shells are completely see-through, and you can even see their hearts beating inside. When scientists started thinking about which animals would be most vulnerable to ocean acidification, pteropods, with their tiny delicate shells, went straight to the top of the list!
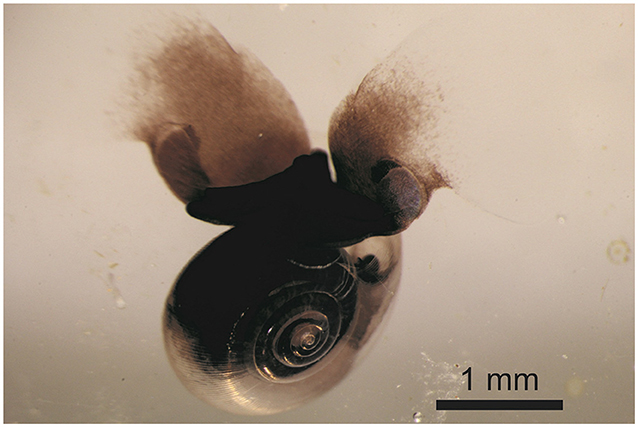
- Figure 1 - A polar pteropod, also called a sea butterfly.
- These microscopic snail flaps the “wings” on their feet like a butterfly, to swim.
Can Sea Butterflies Protect Their Shells?
Researchers have spent years studying sea butterflies. Early research supported their greatest fear: that pteropod shells would simply dissolve in undersaturated water [1]. At that time, the future did not look good for sea butterflies. However, more recent studies found some good news—pteropods are better equipped to deal with ocean acidification than we thought. They have two lines of defense against ocean acidification. The first line of defense is a protective coating on the outside surface of their shells. This layer is like the cling film you might put over a dish of leftovers. It is waterproof and prevents seawater from touching the calcium carbonate. This means that, even if the water is undersaturated, the shell will not dissolve. The only time the shell may be vulnerable is if the protective layer is damaged—like rainwater getting in if your raincoat is torn. For a pteropod, any tear in its protective layer can be dangerous [2].
Shell cracks and scratches are common for pteropods. Pteropods are the prey of sea angels, which are not angels at all from the pteropods’ point of view! Sea angels use their tentacles to grab hold of a pteropod’s shell (Figure 2). They then use their mouthparts to grab the soft body inside the shell and slurp it out. Then they throw away the empty shell like we would do to a candy wrapper. If a pteropod is quick, it can retract far enough inside its shell that the sea angel cannot grab it. In this case, the sea angel gives up and moves on to its next victim. While this pteropod is lucky to have escaped, its shell will show the scars.
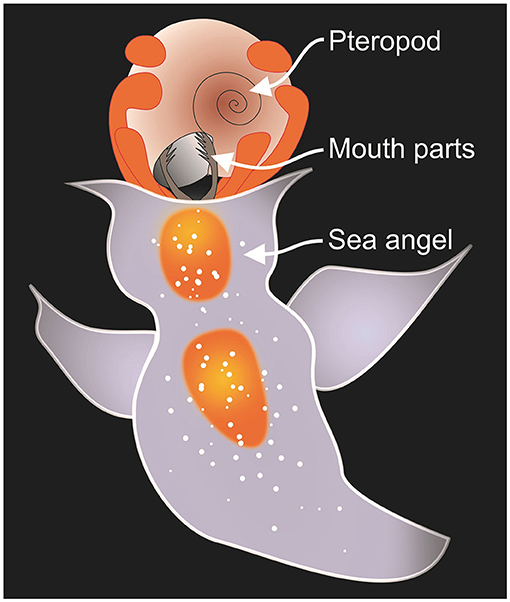
- Figure 2 - A sea angel preying on a sea butterfly.
- If the sea butterfly survives, tears to the protective coating of its shell will expose calcium carbonate to the ocean waters. If the waters are undersaturated, the exposed shell will dissolve [Figure adapted from Lalli and Gilmer [3]].
Wherever the protective coating has been damaged, calcium carbonate is exposed. The pteropod is unable to repair the protective coating, which means that any exposed calcium carbonate will always be vulnerable to dissolution when exposed to undersaturated water. Localized dissolution around a tear is common to sea butterflies living in the polar regions. However, localized dissolution is much more manageable than dissolution of the entire shell!
Can Sea Butterflies Repair Their Shells?
The second line of defense that sea butterflies have against ocean acidification is their ability to repair their shells. While studying sea butterflies with localized dissolution, we noticed something unexpected—it seemed that the damage was deeper than the thickness of an undamaged shell. To understand what was happening, we took 3D X-rays to get a better look at the tiny shells. We saw that an undamaged shell was <10 μm thick (<1/100 of a mm). Where there was dissolution, the shell could be up to three times thicker (Figure 3) [4]. This told us that, while pteropods are unable to repair their shells from the outside, they can make new calcium carbonate and patch themselves up from the inside! This ability to repair their shells is essential to maintaining their homes.
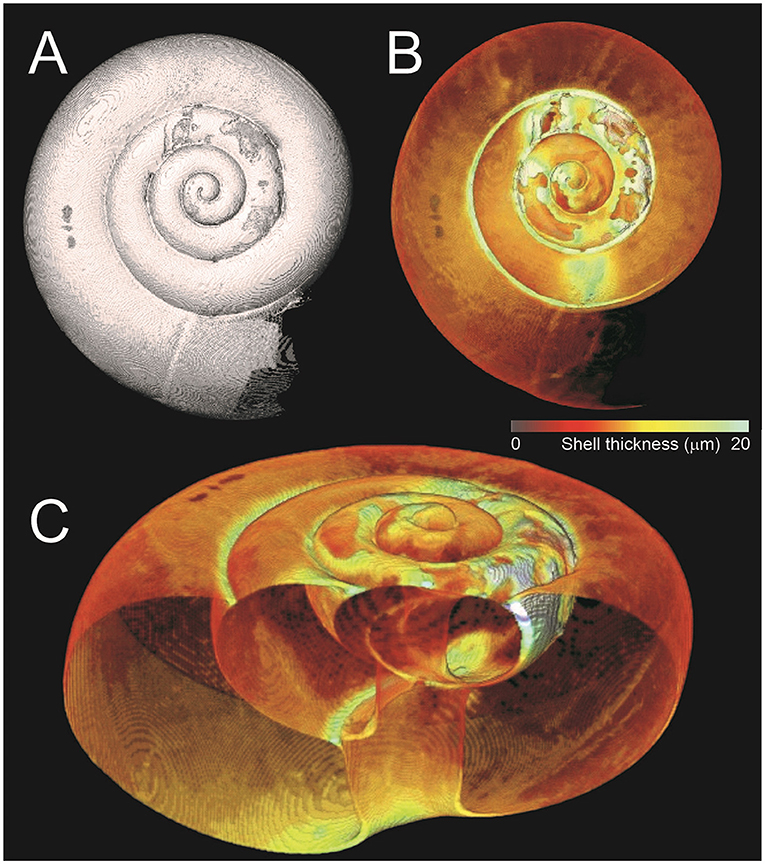
- Figure 3 - Images of a damaged pteropod shell.
- (A) The outer surface of the shell. Note areas of deep damage in the inner whorls. (B) A color map showing the thickness of the shell. (C) The shell from (B) in 3D, showing the inside. Note that the undamaged shell has a uniform thickness (<10 μm). In areas of damage, the shell can be more than 20 μm thick. These are the areas where the pteropod has repaired itself from the inside.
The Future of Sea Butterflies
Their protective coating and ability to patch themselves up from the inside means pteropods are more resilient to ocean acidification than we originally thought. However, the amount of energy they must invest into maintaining their damaged shells comes at a cost—after fixing their shells, some pteropods may not have enough energy for anything else. For example, reproduction may become too much of an effort. It is also important not to think of ocean acidification as happening on its own, because it usually happens along with ocean warming, sea-ice loss, and pollution—all of which could affect ocean animals like pteropods. So, while pteropods may be doing better than expected so far, the true test for these sea butterflies is yet to come.
Glossary
Greenhouse Gas: ↑ When the suns energy is reflected off the earth’s surface certain gases in the atmosphere trap the heat, in the same way as the glass of a greenhouse.
Ions: ↑ Atoms or molecules that have either a positive electric charge as they have lost one or more electrons, or a negative charge as they have gained one or more electrons.
Carbonic Acid: ↑ When CO2 gas dissolves in water (H2O) the two react to form a weak acid called carbonic acid (H2CO3).
Ocean Acidification: ↑ As the world’s oceans absorb fossil fuel derived CO2 from the atmosphere the water becomes acidic.
Undersaturated: ↑ When the concentration of dissolved ions is not as high as it could be. In the case of carbonate ions, carbonate would dissolve in undersaturated waters to increase the concentration of carbonate ions until the water is saturated.
Pteropod: ↑ Meaning “winged foot” in Latin. A microscopic swimming snail found in the ocean. It has evolved to have two wings, rather than a foot, and is commonly called a “sea butterfly.”
Dissolution: ↑ This is the process by which something it broken down into its soluble component parts. In the case of calcium carbonate, dissolution caused by exposure to carbonic acid creates calcium ions (Ca2+) and bicarbonate ions (HCO3−).
Acknowledgments
We are grateful for funding support from NERC, DEFRA, and DECC to the pelagic consortium of the UK Ocean Acidification Programme (Grant no. NE/H 17348/1*).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Original Source Article
↑Peck, V. L., Oakes, R. L., Harper, E. M., Manno, C., and Tarling, G. A. 2018. Pteropods counter mechanical damage and dissolution through extensive shell repair. Nat. Commun. 9:264. doi: 10.1038/s41467-017-02692-w
References
[1] ↑ Bednaršek, N., Tarling, G. A., Bakker, D. C. E., Fielding, S., Jones, E. M., Venables, H. J., et al. 2012. Extensive dissolution of live pteropods in the Southern Ocean. Nat. Geosci. 5:881–5. doi: 10.1038/ngeo1635
[2] ↑ Peck, V. L., Tarling, G. A., Manno, C., Harper, E. M., and Tynan, E. 2016. Outer organic layer and internal repair mechanism protects pteropod Limacina helicina from ocean acidification. Deep Res. II Top. Stud. Oceanogr. 127:41–52. doi: 10.1016/j.dsr2.2015.12.005
[3] ↑ Lalli, C. M., and Gilmer, R. W. 1989. Pelagic Snails. Stanford, CA: Stanford University Press.
[4] ↑ Peck, V. L., Oakes, R. L., Harper, E. M., Manno, C., and Tarling, G. A. 2018. Pteropods counter mechanical damage and dissolution through extensive shell repair. Nat. Commun. 9:264. doi: 10.1038/s41467-017-02692-w
