Abstract
In one of Earth’s largest oceans, the Southern Ocean, tiny organisms called phytoplankton are starving! Not for spaghetti though. How would you like a dinner of iron soup? No? Well, phytoplankton love it. Luckily, these important little organisms can get their iron soup near melting ice, undersea volcanoes, and even near the rear ends of whales! Sounds gross, right? Well, not for tiny phytoplankton. When they are not starving, they multiply enough to be visible from space! They have two super-important jobs that they do for the Earth, for free. First, they produce oxygen for humans and other animals to breathe. Second, they change carbon dioxide into sugar-filled snacks that become food for an organism called krill. And krill are whale food! Phytoplankton do these two jobs while also slowing global warming. Read on to hear how iron soup for phytoplankton might help save our planet!
Phytoplankton’s Superpower: Turning Sunlight Into Sugar
Can you remember the last time you sat down in the grass on a warm summer day, closed your eyes and enjoyed the sun’s energy warming your skin? Have you ever wondered how ocean organisms use that same energy to grow? Sunlight enters the surface ocean and tiny organisms called phytoplankton use that energy, along with nutrients in the water, to do something incredible: they convert carbon dioxide and water into sugar and oxygen, through the process of photosynthesis (Figure 1). That is like bottling sunlight energy! The sugars created by the tiny phytoplankton are eaten by very important marine animals like krill, on which the entire ecosystem, including animals like penguins, seals, sea birds, and whales, depends.
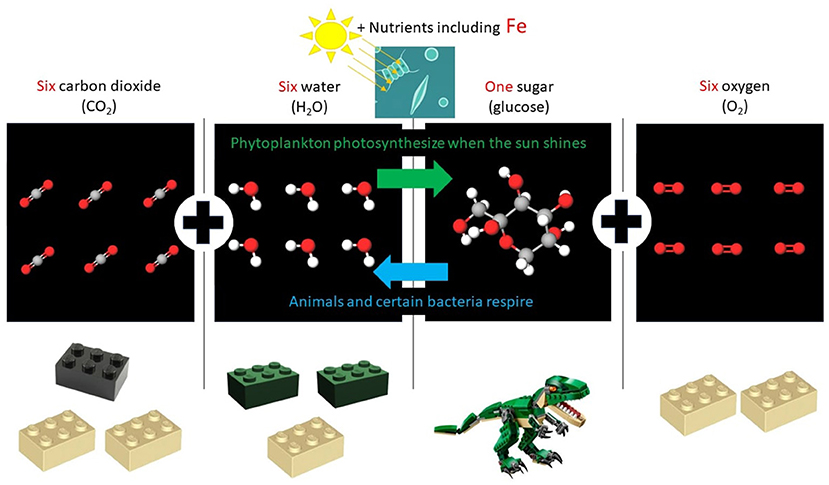
- Figure 1 - By using light as a source of energy, phytoplankton use carbon dioxide and water to make sugar and oxygen.
- This process is called photosynthesis. It is a bit like sticking construction blocks together to make a model. The reverse process is called respiration. In respiration, certain bacteria and animals (like us) get chemical energy by smashing sugar apart, while they breathe in oxygen and breathe out carbon dioxide and water (Images from http://molview.org under a GNU Affero General Public License. LEGO® trademarks can be used in a non-commercial manner so long as we say: LEGO® is a trademark of the LEGO® Group. The LEGO® Group does not sponsor, endorse or authorize this display of LEGO® elements).
Do you like construction blocks? We do! Do you like building models or smashing them apart? If you like building, you are more like phytoplankton; and if you like smashing models apart, you are more like animals and certain bacteria! How? You might make a model of a dinosaur from a pile of construction blocks, while phytoplankton make a “model” of sugar. But their construction blocks are small molecules called carbon dioxide and water (Figure 1). Phytoplankton use sunlight energy to stick their construction blocks together, converting sunlight energy into chemical energy. The more blocks they stick together, the more chemical energy is in the sugar. They collect their sugar molecules inside themselves and use some of the sugar to grow and make more phytoplankton. But when you taste so delicious, everything wants to eat you! Animals and bacteria that eat these sugar-filled phytoplankton smash the construction blocks apart to release the chemical energy, powered by the oxygen they breathe (Figure 1). This is called respiration. Then, the eaters can use that energy to grow. Marine animals cannot construct sugar from sunlight energy, so they need to get sugar by eating phytoplankton, or eat animals that ate phytoplankton. So, if phytoplankton are the ultimate producers, animals and certain bacteria are the ultimate recyclers. Eventually, everything living must die and that is when certain bacteria come in to do the recycling. If there are any nutrients, like iron, mixed in with the sugary snacks, some of that gets recycled too.
Phytoplankton Drive the Ocean’s Biological Carbon Pump
So, phytoplankton produce sugar to grow, and animals and certain bacteria smash the sugar apart again to use the chemical energy to grow. But some of the sugar and dead phytoplankton bits do not get smashed by bacteria or other animals, and these substances sink to the seafloor where they build up, forming sediments. After a very long time, sediments build up more and more and the pressure and temperature rise. If other conditions are just right, the carbon-containing molecules that phytoplankton originally made get rearranged into even longer carbon-containing molecules to form oil or coal. Scientists call the process that “pumps” carbon-rich molecules like sugar deep into the ocean the biological carbon pump (Figure 2). If the carbon pump “runs” long enough, it can move huge quantities of carbon dioxide from the air and the ocean surface into sediments, rocks, or oil.
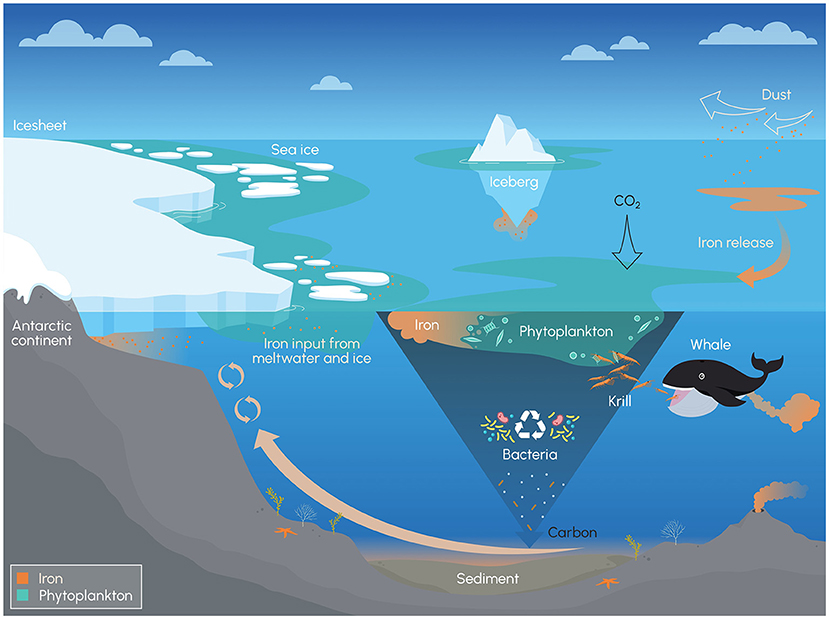
- Figure 2 - When the sun is shining, phytoplankton change carbon dioxide (CO2) into sugar, through photosynthesis.
- When phytoplankton die or get eaten, most of the carbon in the sugar gets recycled by bacteria back into CO2, but some carbon sinks to the ocean floor (dark blue, upside-down triangle). This is the biological carbon pump. In the Southern Ocean, phytoplankton have a difficult time finding enough iron to grow well. Iron can be found near upward currents, melting glaciers, icebergs, or sea ice, and it can also be found downstream of islands and even around krill and whale poo! (Figure credit: Stacey McCormack, Visual Knowledge).
But here is the problem: for the last 200 years, humans have been digging up ancient “bottled sunlight energy”—oil and coal—and burning it! Burning oil and coal releases the chemical energy to heat homes, generate electricity, or power cars. When we burn oil and coal, we smash apart those long carbon molecules, making them into construction blocks again. You might think, “that is great, more construction blocks to play with”, but there is a catch. We have been releasing so much of one construction block, carbon dioxide, and so quickly, that it has built up in the air. Carbon dioxide in the air traps energy that should bounce off Earth back into space. Over time, that extra energy warms the oceans, Earth, and air, and makes it uncomfortable or dangerous for humans and other animals. This is the process behind climate change.
“I Am Starving!” Said The Southern Ocean Phytoplankton
You might be wondering whether the biological carbon pump can help remove carbon dioxide from the atmosphere by pumping it into the deep ocean and sediments…that might slow global warming, right? The answer is: it depends on whether the phytoplankton get a healthy iron soup while the sun is shining [1]. In winter in the Southern Ocean, the days are very short compared to the long dark nights, and there is lots of sea ice blocking the light. So, there is often not enough sunlight in the ocean for photosynthesis. The phytoplankton are also mixed down deep into the dark ocean by strong winds. That all changes in spring and summer though. When sea ice melts, the winds are weaker and the sun shines for longer, and everything is right for a huge phytoplankton party. Unless other ingredients are missing! In addition to light, phytoplankton need a “soup” with plenty of major nutrients and a sprinkle of vitamins and metals such as iron. Phytoplankton need iron to make a whole range of important molecules that allow them to photosynthesize and do other cool tricks. In the Southern Ocean, there are heaps of major nutrients, but the sprinkle of iron is a little harder to find. In fact, most of the time, Southern Ocean phytoplankton are starving for iron!
Thirty years ago, an oceanographer named John Martin came up with an idea [2]. He observed that Southern Ocean phytoplankton had enough major nutrients, but did not grow very well, so he wondered whether they have enough iron. He tested his idea by adding a tiny bit of iron soup to bottles filled with Antarctic seawater. Sure enough, after a few days, phytoplankton grew much better in the bottles that had iron added to them compared to the bottles that did not. Scientists later did the same experiment, but bigger—they added iron soup from the backs of ships directly into the Southern Ocean. Eureka! The phytoplankton grew so well that a patch of ocean became green, and the change in color could be seen from space [3]. All these observations show that the growth of phytoplankton in the Southern Ocean is limited by a lack of iron, and they are screaming “more iron, more iron, please!”
An Iron Soup Shortage?
There are two main reasons why there is not enough iron soup in the Southern Ocean. The first reason is that, in the ocean, iron oxidizes (rusts) and sinks to the bottom unless phytoplankton or certain bacteria get to it in time and take it into their cells or stick special molecules to it. It was not always like this. Before phytoplankton started photosynthesizing 2.5 billion years ago, there was less oxygen and more carbon dioxide in ocean water [4]. So, iron did not oxidize as quickly, and phytoplankton had more time to bump into it. Through the biological carbon pump, and with some recent help from trees, phytoplankton have reduced the carbon dioxide and increased the oxygen in our oceans and air. Today, iron is only abundant close to its sources (Figure 2), before it oxidizes and is lost to the deep, dark ocean depths.
The second reason for the iron soup shortage is that the Southern Ocean is isolated. Wind and surface ocean currents whip around Antarctica, isolating it from many of the nearby sources of iron. For instance, dust blown off deserts is a big source of iron for other oceans, but very little dust blows into the Southern Ocean. In fact, the air over the Southern Ocean is some of the cleanest in the world. Deep ocean currents can bring iron up to the surface near the Antarctic continent, and melting ice can deliver iron into the ocean water (Figure 2). However, over most of the Southern Ocean, this is never quite enough to allow phytoplankton to have the giant party they are capable of when iron is plentiful!
How Can You Help?
Climate change is warming our air and our oceans, melting ice, changing wind and ocean current patterns, and making ocean water more acidic. You can help by becoming a scientist and studying how climate change may also change the amount of iron soup available to Southern Ocean phytoplankton. Maybe extra ice melting in Antarctica will provide more iron soup for phytoplankton? Maybe extra phytoplankton will allow more krill to grow, and more whales can feed on the krill? Maybe more iron means the biological carbon pump will move more carbon dioxide into sediments and help slow global warming? There are many questions, and we need young scientists like you to help figure them out! Will you take on this challenge?
Glossary
Phytoplankton: ↑ Microscopic aquatic organisms that obtain their energy through photosynthesis exactly like plants on land.
Photosynthesis: ↑ The way that plants and phytoplankton grow by using light to transform carbon dioxide and water into sugar and oxygen when they have enough nutrients.
Krill: ↑ Tiny shrimp-like creatures commonly found in polar waters. They are important food for whales and penguins.
Chemical Energy: ↑ A form of energy stored in molecules that can be released when they are transformed into smaller molecules during a chemical reaction like respiration or burning fossil fuels.
Respiration: ↑ Breathing in and out; how animals get oxygen and releases carbon dioxide gas.
Biological Carbon Pump: ↑ A process in which carbon is moved from the air into the deep ocean for a very long time by living organisms like phytoplankton and bacteria.
Oxidation: ↑ When something reacts with oxygen, often causing it to change or rust. Like when metal turns orange.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
PM and DL were supported by the Australian Research Council (ARC) FT190100688. DL received grant funding from the Australian Government as part of the Antarctic Science Collaboration Initiative program (project ID ASCI000002). This research was also supported by the Australian Research Council Special Research Initiative, Australian Centre for Excellence in Antarctic Science (Project Number SR200100008).
References
[1] ↑ Smetacek, V., Klaas, C., Strass, V. H., Assmy, P., Montresor, M., Cisewski, B., et al. 2012. Deep carbon export from a Southern Ocean iron-fertilized diatom bloom. Nature 487:313–9. doi: 10.1038/nature11229
[2] ↑ Martin, J. H. 1990. Glacial-interglacial CO2 change: The iron hypothesis. Paleoceanography 5:1–13. doi: 10.1029/PA005i001p00001
[3] ↑ Boyd, P. W., Jickells, T., Law, C. S., Blain, S., Boyle, E. A., Buesseler, K. O., et al. 2007. Mesoscale iron enrichment experiments 1993–2005: synthesis and future directions. Science (80- ) 315:612–7. doi: 10.1126/science.1131669
[4] ↑ Lyons, T. W., Reinhard, C. T., Planavsky, N. J. 2014. The rise of oxygen in Earth’s early ocean and atmosphere. Nature 506:307–15. doi: 10.1038/nature13068
