Abstract
What would you do if you had a disease, but the treatment made you feel even more sick? Chemotherapy drugs are a common tool used to treat cancer, but they have negative side effects. These drugs change the cells in our body leading to pain or burning or numbness in the fingers and toes. These drugs also impact cancer patients’ lives and limits their much-needed chemotherapy treatment. Our research investigated how cells and their DNA contribute to this pain. When we are hurt, certain cells send signals that create pain to help us heal. Studying these cell’s DNA could help us learn why cancer treatment pain occurs, and how we can possibly prevent it. While comparing normal mice to mice that have had their DNA changed, we explore how cancer treatment pain develops, and how certain cells are affected by chemotherapy.
What Is Cancer Treatment Pain?
Chemotherapy drugs, a form of cancer treatment, are an important tool for battling cancer. They can kill cancer cells and extend a patient’s lifespan, or even cure them. However, chemotherapy drugs also have unintended consequences because they cannot tell the difference between cancer cells and healthy ones [1]. They can cause hair loss, blood problems, weight loss, and digestive issues. But what we are interested in is how they hurt our nerves. Nerves are really important because they allow our brains to talk to and control our bodies. Without them, we would not be able to move or feel anything! It is actually possible that men and women’s nerves talk to each other differently, which is an idea we explored in our study. The nerve pain we have been talking about happens when chemotherapy damages the nerves in the hands and feet, preventing them from sharing important information (Figure 1). This damage leads to pain, burning, or a tingling sensation like the one you experience when your foot “falls asleep.” Imagine feeling like your foot was asleep all the time! You probably would not feel like doing anything, much less taking the medication that were making you feel that way! That is why these symptoms often prevent patients from continuing the chemotherapy treatment they need to control their cancer [2]. The worst part is there is no known cure for this nerve pain. Our research tries to find out if we can shut down communications between cells that cause nerve pain to bring us closer to changing that.
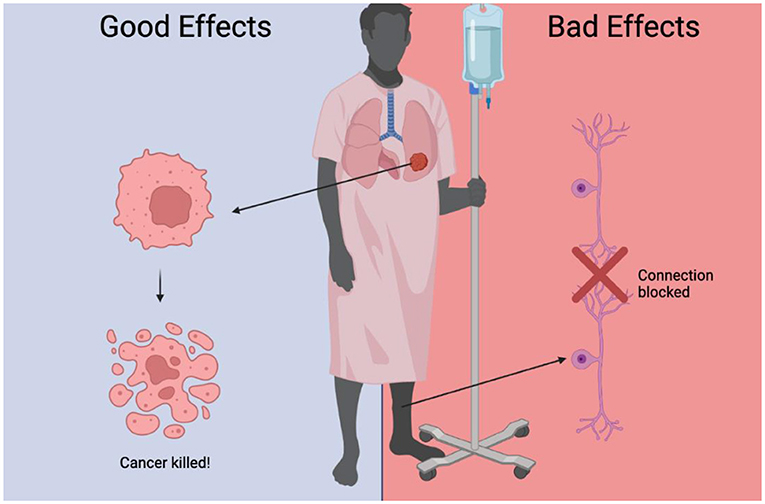
- Figure 1 - Chemotherapy kills cancer cells, but it can also have unwanted side effects.
- It disrupts the connections between cells, causing pain, and over activates certain populations of immune cells, resulting in inflammation.
The Major Players
If our mission is to shut down miscommunication between pain-causing cells, then we need to know who is involved in this operation and what they are up to. Our bodies sense pain with special nerve cells. You can think of these pain sensor cells as hall monitors who are vigilantly looking for signs of discontent. When they get complaints from the student body, they rush to report them to the student council. In this analogy, the student council represents clusters of cells that relay pain information to the brain. The council members decide how and when they communicate these reports to the principal so action can be taken! But sometimes, the hall monitors become overly sensitive, taking tips from faulty sources. The student council does not know this and sends the information through anyway. This confusion causes changes that do more harm than good. In the same way, inflammation causes pain sensor cells to overreact to harmless things, causing unnecessary pain. It is like the class tattletale overwhelming the hall monitor with rumors! It makes sense that inflammation makes us more sensitive to pain. After all, your forehead is much more likely to be painful when there is a swollen bump on it! Inflammation is a major driver in nerve pain and is caused by our immune cells when they respond to damage or injury from chemotherapy drugs. This makes pain sensor cells and immune cells, the major players in our study.
But how and why do immune cells cause inflammation and make us more sensitive to pain after chemotherapy? These cells communicate with pain sensor cells by releasing chemical signals that are “pain causing.” Cells make these proteins based on a DNA blueprint. For our study, we focused on how the instructions in the blueprint are producing pain in these poor patients. Picture DNA like a set of connector-blocks. New blocks can attach to the current set of blocks. This changes the block design, and therefore its function. When a particular combination of blocks is made, it changes a cell’s shape, and turns it into a mean, pain-causing machine! We set out to discover if we could decrease pain caused by chemotherapy drugs by preventing these blocks from morphing into a different shape. We decided to test this by using mutant mice. That is right, we were able to breed mice that did not have this ability to change shape in their blueprint. We then compared them to normal mice who naturally have this ability. Changing the DNA blueprint and looking at the effects can help us understand what specifically causes nerve pain after chemotherapy.
What We Did
Our experiment used normal mice and mice with modified DNA, giving them new blueprints that will not allow for cells to respond and cause pain (mutant mice). Neither group had cancer [3]. We gave them a chemotherapy drug and measured their pain response in several different ways. You know that face you make when you stub your toe? Mice grimace when they are in pain, just like humans do. Looking at their “body language” allows us to measure the amount of pain they are in before we do anything to them. We also tested their sensitivity to evoked pain, or pain caused by something we did. We put them on a slightly hot surface (not hot enough to hurt them and cause permanent damage!) and timed how long it took them to react to the heat. The more pain they are in, the faster they react. When they react faster than we expect them to, this tells us that there may be problems with how the cells are communicating. We also looked at how they reacted to touch by applying pressure to their hind paws with a stiff wire. Like the heat, a quicker response demonstrates a higher amount of pain.
So, What Happened?
We found that mutant mice grimaced less and were less sensitive to touch compared to normal mice. Mutant male mice were less sensitive to heat after chemotherapy treatment. This suggests that this gene is responsible for driving sensitivity to heat after chemotherapy. In addition to their behavior, we looked at the immune cells that are communicating with the cell clusters that are sending information to the brain, which no previous studies have investigated. As expected, chemotherapy caused more immune cells to be activated and sent to those cell clusters, making pain sensor cells more sensitive (Figure 2). Going back to our previous example, chemotherapy unleashes the “tattletales,” making the hall-monitors more sensitive and overloading the student council with false information. However, the mutant mice had less active immune cells with chemotherapy, with a marked difference found in females. This means changing the blueprint was helpful!
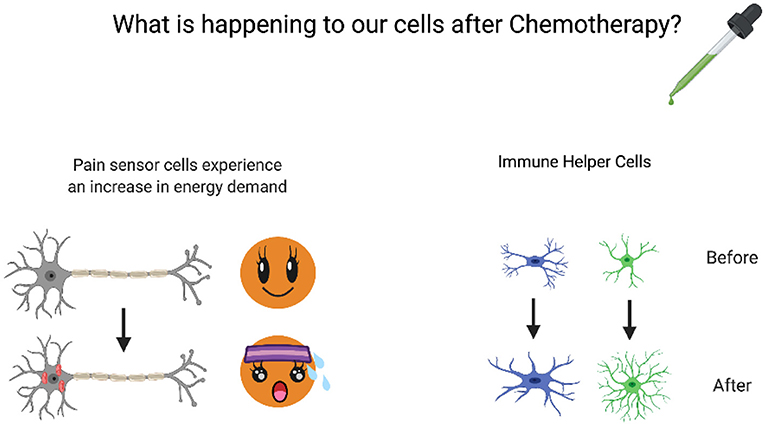
- Figure 2 - Nerve pain from chemotherapy is associated with a variety of effects on different cell types.
- It causes pain sensor cells and immune helper cells to go into “action mode” and increases the energy demand of pain-sensing cells. The immune helper cells change in appearance, becoming more “branchy.”
We already know that chemotherapy is known to damage nerve cells. Our experiment showed that the chemotherapy drug contributed to an increase in signs of cell damage in nerve cells, regardless of sex or mutation. This finding matches our other data. It makes sense that mice with damage in the cells that relay pain are in pain when they are not supposed to be, does not it? Finally, we looked at how much energy pain sensor cells use. This was measured by how much oxygen they consume. Just like you breathe harder when you exercise, cells use more oxygen when they are active. Basically, we want to know if pain sensor cells are working harder to survive after being treated with chemotherapy. Chemotherapy increased the cells’ oxygen consumption in all groups of mice, confirming that it increased energy demand. However, oxygen consumption was lower in the mutant mice, suggesting that the mutation helps decrease sensitivity.
Our next step was to understand the immune cells and helper cells that are involved in nerve pain. The normal mice had more active helper cells after chemotherapy, which makes sense considering that they were more sensitive to pain, requiring more help! The mutants’ helper cell activation was relatively lower, which could be a potential explanation for their decreased pain sensitivity. Mutants had fewer inflammatory immune cells and more cells involved in long-term immunity. One explanation of our findings could be that changing the blueprint leads to the activation of long-term immune cells, which help our bodies stay nice and healthy.
Why It All Matters
After all this hard work of breeding mutant mice and running experiments, we found some important takeaways that could make a difference for chemotherapy patients! Our experiments explored how chemotherapy drugs impact pain sensitivity, and how our mutation affected this. As expected, we saw that normal mice of both sexes were more sensitive to touch and heat and grimaced more after chemotherapy. This means that they were experiencing pain, just like humans who undergo chemotherapy treatment, and tells us that the mice were a good model for our study, the mutant mice were less sensitive to touch and heat and grimaced less. The fact that they were more tolerant to touch and heat and appeared less uncomfortable after chemotherapy tells us that the mutant mice were protected from chemotherapy pain!
The results of our experiments also reveal a sex difference. Mutant males had almost no change in their natural pain response after chemotherapy. Female mutant animals were more sensitive than the males, but significantly less sensitive than the normal female mice. So even though they experienced pain, it was still an improvement from the normal female mice’s pain. All these results suggest that changing the blueprint worked! However, there might be differences in how it works in males and females. Figuring those out could help us better personalize our treatment for nerve pain. Now, that would be an exciting idea to explore in future experiments!
We also evaluated energy use in pain sensor cells. When exposed to chemotherapy, the pain sensor cells used more oxygen, indicating stress. Interestingly, this finding was the same in the normal mice and the mutants! This may imply that unknown factors could be affecting the changes to pain sensor cells observed after chemotherapy. Discovering this mystery could go a long way toward the early detection and prevention of nerve pain in both sexes. Overall, our results show that the type of blueprint is an important determinant of whether nerve pain will occur and a good target for continued research. Understanding what nerve pain looks like on a cellular level and what might be causing it is an important step toward a future in which cancer patients can recover without debilitating chemotherapy pain. Who knows, maybe you will be the researcher to uncover new and exciting discoveries about cancer treatment someday!
Glossary
Inflammation: ↑ A state of immune activation caused by illness or injury that can heighten sensations of pain.
Immune Cells: ↑ Cells that are responsible for defending the body from outside attackers like bacteria and viruses. When overactivated, as in the case of chemotherapy pain, they can do more harm than good.
DNA: ↑ The genetic blueprint that cells use to know how to function. DNA can be modified to change the cell’s behavior.
Mutant Mice: ↑ Mice that have had their DNA changed in order to explore the effects of certain genes.
Sensitivity: ↑ How strongly an animal responds to a particular sensation (such as pain).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This research was funded by the NIH/NIDDK [grant number DK130015 (MB), NIH/NIGMS, grant number GM147094], the University of Texas System STARS Program Research Support Grant (MB), and the Rita Allen Foundation Grant (MB). We would like to thank Charmi Porwal and Prapti H. Mody for their contributions to the early phases of the manuscript. We would also like to thank all current and former lab members for their input on this manuscript. Graphics created with Biorender.com.
Original Source Article
↑Agalave, N. M., Mody, P. H., Szabo-Pardi, T. A., Jeong, H. S., and Burton, M. D. 2021. Neuroimmune consequences of eIF4E phosphorylation on chemotherapy-induced peripheral neuropathy. Front. Immunol. 12:642420. doi: 10.3389/fimmu.2021.642420
References
[1] ↑ Argyriou, A. A., Bruna, J., Genazzani, A. A., and Cavaletti, G. 2017. Chemotherapy-induced peripheral neurotoxicity: Management informed by pharmacogenetics. Nat. Rev. Neurol. 13:492–504. doi: 10.1038/nrneurol.2017.88
[2] ↑ Beijers, A., Mols, F., Dercksen, W., Driessen, C., and Vreugdenhil, G. 2014. Chemotherapy-induced peripheral neuropathy and impact on quality of life 6 months after treatment with chemotherapy. J. Community Support Oncol. 12:401–6. doi: 10.12788/jcso.0086
[3] ↑ Agalave, N. M., Mody, P. H., Szabo-Pardi, T. A., Jeong, H. S., and Burton, M. D. 2021. Neuroimmune consequences of eIF4E phosphorylation on chemotherapy-induced peripheral neuropathy. Front. Immunol. 12:642420. doi: 10.3389/fimmu.2021.642420