Abstract
There is nothing like eating a fruit when it is just ripe. Ripeness brings out the best flavor, texture, and even an appetizing smell. Eat that same fruit a week before it is ripe, and you will get a completely different experience. The ripeness of fruit influences the choice of which fruit we pick at supermarkets. However, once fruits are ripe, they tend to spoil quickly, as you might have noticed in your own experience. This article provides an insight into two of the changes that are associated with fruit ripening: (1) softness; and (2) flavor, in particular, sweetness, and the role of ethylene gas in controlling fruit ripening.
Before We Look at How to Control Fruit Ripening, Let Us See How Fruits Ripen
As fruit-bearing plants grow, the fruits accumulate water and nutrients from the plant and they use these nutrients to create their flesh and seeds. Most growing fruits initially provide protection to the developing seeds. At this stage, fruits are generally hard and unattractive to predators—including us! After seed development and fruit growth, the properties of the fruit change to make the fruit more attractive to potential consumers, such as animals, birds, and humans [1]. These changes include the most common ways by which we judge whether a fruit is ripe or not, including external features, such as softness to the touch, and internal features, such as sweetness. Fruits also change color as they ripen. This happens because of the breakdown of a green pigment called chlorophyll, along with the creation and accumulation of other pigments responsible for red, purple, or blue hues (anthocyanin), or bright red, yellow, and orange hues (carotenoids), to name a few.
First, how is fruit softness regulated? The softness or firmness of a fruit is determined by the state of its cell walls. Cell walls surround each plant cell and they consist of a rigid layer of sugars, called polysaccharides, which encase each cell’s plasma membrane (Figure 1). The three main polysaccharide of the cell wall are cellulose, hemicellulose and pectin. Cellulose is made up of hundreds of glucose sugars joined together to form a long chaiin; hemicelluloses are also long chains of sugars, but unlike cellulose, these can include many different types of sugar, such as glucose, xylose, galactose, and mannose and instead of being linear are branched structures; pectins are also long branched chains of sugars, but in this case the sugars are galacturonic acid, rhamnose, galactose, and arabinose. As the cell wall begins to break down, the fruit starts to get softer [2]. Cell wall breakdown happens when proteins called enzymes dissolve these important cell wall polysaccharides. The activity of these enzymes is directly linked to the shelf life and texture of the fruit [2]. Fruit softness is also affected by the fluid pressure inside the plasma membrane (called turgor pressure). Turgor pressure keeps the fruit firm, just like air pressure inside a balloon keeps the balloon firm. After maturation or harvest, fruits lose fluid (water), causing a decrease in turgor pressure, so the fruits shrivel. In fruits like strawberries, once the fruit loses 6–10% of its fluid, it no longer looks good and might not be picked up by consumers.
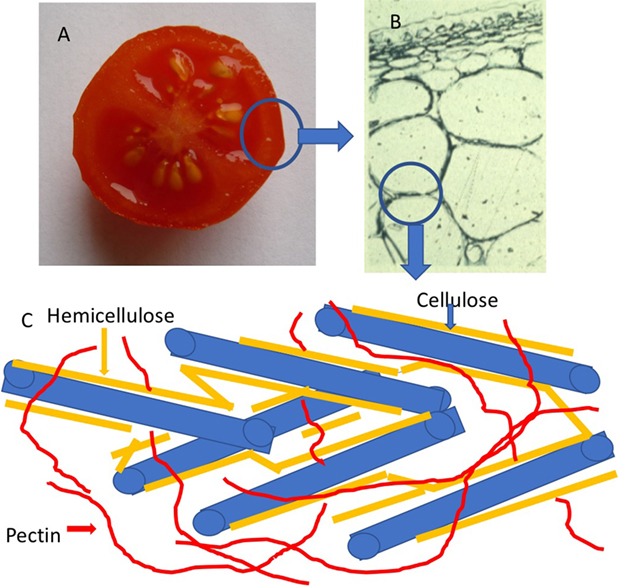
- Figure 1
- Plant cell wall structure. A. The cell wall structure of a tomato fruit A. can be viewed under a light microscope B. The cells can be seen to be surrounded by a polysaccharide cell wall, which is seen in the blue circle. C. The cell wall is composed of three main components, called cellulose, hemicellulose, and pectin.
Let us now discuss how fruit ripening brings out the flavor of fruit—particularly a fruit’s sweetness. During ripening, there is an increase in the breakdown of starch inside the fruit, and a corresponding increase in the amount of simple sugars which taste sweet, such as sucrose, glucose, and fructose. This process is particularly obvious in bananas as they ripen. Green bananas do not taste sweet at all, and the riper they get, the sweeter they taste. There is also a decrease in acidity as the fruit ripens and a decrease in bitter plant substances, such as alkaloids. Last, as fruits ripen they produce complex compounds that are released into the surrounding air, giving a ripe fruit its pleasant aroma.
Through these changes, fruits ripen and become sweet, colored, soft, and good-tasting. It is good for the plant to invest its resources into the fruit and its ripening because a ripe fruit attracts the consumers that help the seeds to be spread far and wide, which is important for the plant’s survival and regrowth.
How Can We Stop Fruits from Ripening During Storage and Transport?
A major concern with ripened fruit is that it does not last very long before it begins to spoil. The loss of firmness and the production of sugars associated with ripening can also make the fruit susceptible to pathogens like bacteria and spoilage. Over-softening of fruit is a major cause of spoilage during transportation, particularly for tropical fruits, such as mangoes and bananas. Spoilage can be reduced by rapid transportation of fresh fruits, or by slowing down fruit ripening. There are several ways to slow down fruit ripening. One way to slow down ripening is by lowering the temperature. Cold temperatures above freezing are usually used. Even though all fruit can be frozen, upon thawing many fruits lose their flavor and their texture and become very mushy. Raspberries are a possible exception—they can often be found frozen in the grocery store. Normally, to freeze fruit, the fruit is first cut into small pieces and when thawed, these pieces can be used to make purees or smoothies. The good news is that freezing tends to retain the nutritional value of the fruit. Several fruits, such as bananas, can be damaged by chilling and this limits this approach [2]. That is why we do not put bananas in the fridge! Another way to slow down ripening is by controlling the atmosphere around the fruit, primarily by increasing carbon dioxide levels and reducing oxygen levels. Fruit need oxygen to ripen, so if there is less oxygen in the atmosphere, the fruit will ripen more slowly. One final way to slow down ripening is to block the action of ethylene. Ethylene is a hormone required to trigger fruit ripening, and it can be blocked by using synthetic compounds, such as 1-methyl-cyclo-propene (1-MCP). 1-MCP is also used to maintain the freshness of cut flowers.
Ethylene Gas Can be Used to Regulate Fruit Ripening
Ethylene is a gas and is known as the “fruit-ripening hormone.” Every fruit has a certain level of ethylene production throughout its lifecycle. However, in some fruits, ethylene levels shoot up when the fruit starts ripening. Based on their response to ethylene during maturation, fruits can be classified into two major groups. The first group is called the climacteric fruits, in which ripening are accompanied by a burst of ethylene. These fruits can also respond to external ethylene by increasing their ripening rate. These include fleshy fruits, such as tomato, avocado, apple, melon peach, kiwi, and banana. The second group is called the non-climacteric fruits, in which ethylene production does not increase during ripening. However, these fruits can still ripen if they are exposed to an external ethylene source, such as a ripening climacteric fruit. These include strawberry, grape, and citrus fruits [3]. We will focus on ripening of climacteric fruits that are influenced by ethylene.
For climacteric fruit, exposure to an initial, small concentration of ethylene causes the fruit to produce greater quantities of ethylene until a peak concentration is achieved [4]. This increase in ethylene concentration triggers an increase in the fruit’s metabolism and causes the changes to the fruit that occur during ripening. Ripening of climacteric fruits can, therefore, be slowed down by reducing the amount of ethylene the fruits make or by blocking ethylene’s actions [5]. The methods we described above for slowing down ripening work in this way, because, in general, low temperatures reduce metabolism in fruit. Controlled atmospheres limit the amount of oxygen around the fruit, and oxygen is needed to make ethylene. Ethylene action is inhibited by carbon dioxide and by 1-MCP. Another method for slowing down ripening is to remove ethylene from the storage environment by using materials that absorb ethylene, such as potassium permanganate. Once the fruit reaches its destination, it can be ripened by exposure to ethylene gas.
The effect of ethylene on ripening is dependent on many factors. The fruits need to be mature enough to be able to respond effectively to ethylene. In highly sensitive species, like cantaloupes or bananas, ripening is immediately stimulated by ethylene, but the more immature the fruit, the greater the concentration of ethylene required to cause ripening. In the less sensitive species, like tomatoes or apples, ethylene treatment reduces the time before ripening occurs. Some fruits, such as avocados, do not ripen while attached to the tree and gradually increase their sensitivity to ethylene with time after harvest [6].
Why Does a Rotten Apple Spoil the Whole Basket? How Can This Knowledge Help Us?
All plants produce some ethylene during their life cycle. Ethylene production can increase up to 100-fold or more during particular stages—for instance in response to a wound [1]. Ancient Egyptians used to cut figs to enhance their ripening, since ethylene produced by the injured fruit tissue triggered the ripening response. Similarly, the ancient Chinese used to burn incense in closed rooms with stored pears, because ethylene was released as a by-product of the burning incense. The saying, “one bad apple can spoil the whole basket,” is based upon the release of ethylene from rotting apples, which accelerates the ripening of other apples around the rotting one [5].
Ethylene gas is commercially used to ripen fruits after they have been picked. Fruits, such as tomato, banana, and pear are harvested just before ripening has started (typically in a hard, green, but mature stage). This allows time for the fruit to be stored and transported to distant places. Once the fruit reaches its destination, ripening is conducted under controlled conditions. This is usually carried out in specially constructed ripening rooms, with optimum ripening temperature, humidity, and ethylene concentration. These special conditions cause the fruit to ripen at a consistent rate. In supermarkets, you might come across these fruits as “Ripe ‘n’ Ready” [5]. Usually, low concentrations of ethylene are used commercially for fruit ripening, because that is all it takes to stimulate the fruit’s natural ripening response. By the time the ethylene-treated fruit reaches the consumer, the commercially applied ethylene is gone, and the fruit is producing its own ethylene. Both ethylene and another widely used ripening agent, methyl jasmonate, are reported to be non-toxic to humans; however, they are relatively expensive.
Try This at Home!
Understanding the effects of ethylene on fresh produce can be helpful in ripening fruits in our own kitchen.
- If you have an unripe avocado or other fruits at home, try putting them in a paper bag with a ripening banana. This will speed up the ripening of the avocado because ethylene emitted by the ripening banana will trigger the climacteric response in the avocado. This strategy works best when the ripening fruit is one that emits a high concentration of ethylene, such as an apple, pear, banana, or passion fruit [5].
- Try putting a green lemon with a ripening banana in a paper bag, as above, and see what happens to the color of the lemon. Ethylene is also used to “de-green” citrus, by triggering the breakdown of the green pigment (chlorophyll), resulting in orange and yellow coloration of the peel. No loss of flavor is caused because this is merely a continuation of the natural plant process.
If you try these things, keep in mind that ripening is best conducted at room temperature, around 20°C, because low temperatures can inactivate important fruit-ripening enzymes. So, it is best to try this outside of the refrigerator.
Glossary
Cell wall: ↑ A complex structure, consisting mainly of polysaccharides, that surrounds plant cells and provides their structure and rigidity.
Polysaccharide: ↑ A molecule composed of long chains of sugars such as glucose joined together to form either linear or branched chains.
Cellulose: ↑ A polysaccharide found in the cell wall composed of long linear chains of glucose.
Hemicelluloses: ↑ A group of polysaccharides found in the cell wall. They are long branched chains of sugars which commonly include glucose, xylose, arabinose, galactose, and mannose.
Pectin: ↑ A group of polysaccharides found in the cell wall. They are long branched chains of sugars which commonly include galacturonic acid, rhamnose, galactose, and arabinose.
Ethylene: ↑ A gas (C2H4) produced by plants, and known as the “ripening hormone,” which stimulates fruit ripening.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
[1] ↑ Grierson, D. 2013. “Ethylene and the control of fruit ripening,” in The Molecular Biology and Biochemistry of Fruit Ripening, eds Grahman, S., Mervin, P., James, G., and Gregory, T. (Boston: Blackwell Publishing Ltd), 43–73.
[2] ↑ Osorio, S., Fernie, A. R. 2013. “Biochemistry of fruit ripening,” in The Molecular Biology and Biochemistry of Fruit Ripening, eds Grahman, S., Mervin, P., James, G., and Gregory, T. (Boston: Blackwell Publishing Ltd), 1–19.
[3] ↑ Alexander, L. 2002. Ethylene biosynthesis and action in tomato: a model for climacteric fruit ripening. J. Exp. Bot. 53, 2039–2055. doi:10.1093/jxb/erf072
[4] ↑ Mazonde, B., Mujuru, F., and Muredzi, P. 2017. Design of a controlled atmospheric storage facility for climacteric fruits. Int. J. Rural Dev. Environ. Health Res. 1, 47–59.
[5] ↑ Frontline Services. 2015. Fruit Ripening Gas – Ethylene. Available at: http://www.frontlineservices.com.au/Frontline_Services/Fruit_ripening_gas_-_ethylene.html (Accessed: November 2, 2017).
[6] ↑ Brady, C. J. 1987. Fruit ripening. Annu. Rev. Plant Physiol. 38, 155–178. doi:10.1146/annurev.pp.38.060187.001103