Abstract
Climate change is one of the biggest problems our planet faces, caused by too many greenhouse gases (GHGs) in the atmosphere. The most important GHG produced by humans is carbon dioxide (CO2). To slow down global warming, we need to reach net zero CO2 emissions by balancing the amount of CO2 we release with the amount we remove. One exciting way to help us to do this is by turning CO2, often seen as waste, into useful products and energy. To make this possible, scientists use special substances called catalysts. In this article we show how some amazing materials like zeolites and OMS (ordered mesoporous silica) can act as catalysts to help transform CO2 into valuable products and energy, opening up a new way to protect our planet and helping to fight climate change.
Role of Greenhouse Gases
Greenhouse gases (GHGs) in the atmosphere help control Earth’s temperature and climate. They act like a blanket, keeping Earth warm enough for life (Figure 1A). Without GHGs, the Earth would be freezing, and humans, animals, and plants would not be able to survive.
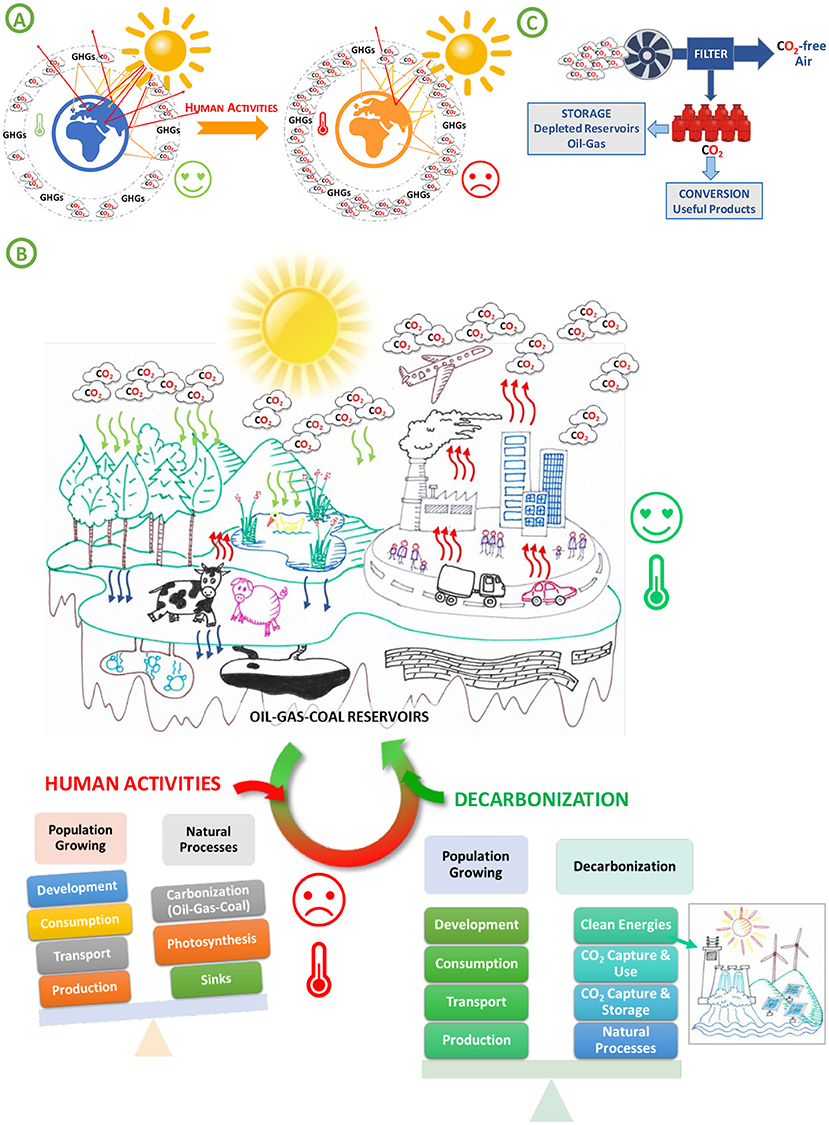
- Figure 1 - (A) GHGs in the atmosphere help to control the Earth’s temperature.
- (B) Human activities like consumption, production and transportation are adding more and more CO2 to the atmosphere, leading to an increase in the global Earth’s surface temperature. Balancing the amount of CO2 is possible by decarbonization processes that allow a return to a favorable global Earth’s surface temperature. (C) Decarbonization process involves taking directly CO2 from air or from the fumes released by factories. This CO2 can be stored underground or used as a building block to create useful products (Figure credit: Pablo A. Ramos).
The main GHGs are water vapor (H2O), carbon dioxide (CO2), and methane (CH4). These gases have naturally existed for millions of years, and they all play a role in making life possible. However, human activities like consumption such as the use and such as the use and spending of goods, services and activities in order to satisfy human needs, production of goods in factories, and many forms of transportation are adding more and more GHGs to the atmosphere (Figure 1B). This leads to increase global Earth’s surface temperature influencing on climate change, which harms ecosystems, reduces biodiversity, and causes problems like acidic ocean waters, which threaten the entire food chain (Figure 1A).
CO2: The Main Greenhouse Gas
CO2 is the most important GHG produced by humans. It remains in the atmosphere for a long time, hundreds or even thousands of years. Natural processes called carbon sinks can remove CO2 from the air (Figure 1B). For example, photosynthesis, which is the way plants absorb CO2 and convert it into oxygen and food, is a carbon sink. Natural absorption of CO2 by the oceans and geological processes such as absorption of CO2 by rainwater or reaction with rocks and soil to form new minerals over thousands of years are other examples of carbon sinks.
Unfortunately, however, these natural processes are not fast enough to absorb the additional CO2 that humans are continually adding to the atmosphere.
We Need to Act Now!
Scientists agree that to stop global warming, we must reach what is called net zero CO2 emissions by 2050. Net zero means balancing the amount of CO2 we release with the amount we remove from the atmosphere, so no extra CO2 builds up. But reaching “zero emissions” is extremely difficult. Think about how much we depend on cars, planes, factories, and even electricity, which often generate CO2. So, we have to explore solutions to remove CO2 that is already in the air.
Decarbonization Approaches
Scientists have been working for several decades on various strategies to reduce CO2 emissions and to remove it from the atmosphere—a process known as decarbonization (Figure 1C). One approach is based on directly capturing CO2 from the fumes released by factories and storing those gases, in a process called sequestration. For CO2 storage, permanent reservoirs are used, such as deep underground geological formations, like depleted oil and gas reservoirs or saline aquifers. Another method of decarbonization is to plant trees to create woodlands or to replant forests that have been cut down.
Furthermore, over the last 20 years, scientists have been exploring an exciting new method of decarbonization: using CO2 as a building block to create useful products and energy.
Why Use CO2 to Make Useful Products?
Carbon (C) is one of the most important building blocks of life. It is found in all living or formerly living things, like plants, animals, and even in your food. Carbon is also in many man-made products we use every day, like plastics, furniture, clothing, paints, medicines, cosmetics, and fuels. What makes carbon so special is its ability to form strong bonds with itself and with other elements like oxygen (O), nitrogen (N), and sulfur (S). These strong bonds allow the creation of all kinds of natural and man-made materials (Figure 2A).
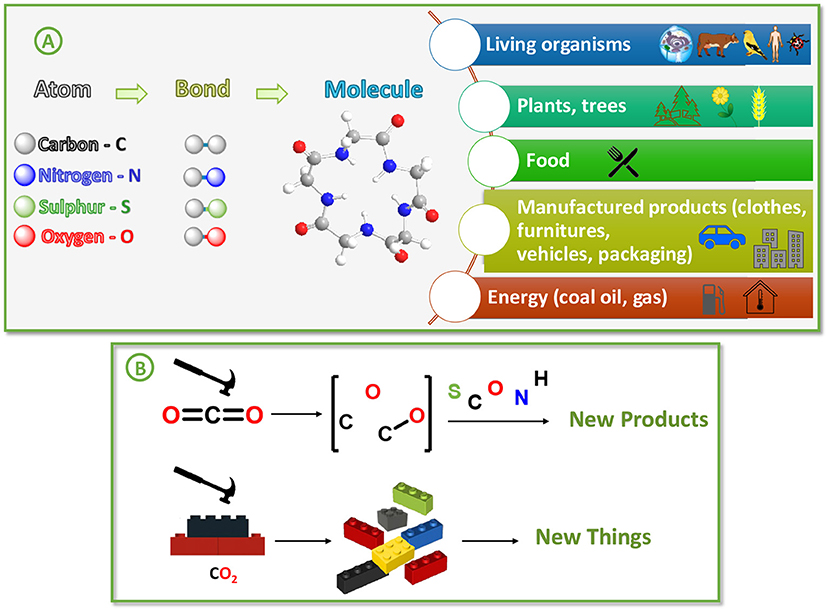
- Figure 2 - (A) Carbon can form strong bonds with other elements, creating many natural and man-made products.
- (B) Breaking the strong bonds of CO2 to free up the carbon and oxygen atoms can allow those atoms to be recombined to produce new products. You can think of this like breaking apart Lego pieces to create new structures.
CO2, the gas we usually think of as waste, is actually a great source of carbon. Therefore, scientists are finding ways to take the carbon locked up in CO2 and use it to make useful things (Figure 2B).
Why is it Hard to Transform CO2?
CO2 is a super stable molecule. Its carbon (C) and oxygen (O) atoms are tightly bonded together, like LEGO pieces firmly clicked together. Breaking these bonds and rearranging the atoms to create new molecules requires a lot of effort, which means that the chemical transformation of CO2 into something new is a big challenge. To overcome this barrier and make this process easier and faster, chemists use powerful tools called catalysts.
Catalysts Can Help With Decarbonization
A catalyst is a substance that speeds up chemical reactions without being used up or changed. Catalysts encourage molecules to lock together and form new products by reducing the efforts needed for the reaction. Catalysts have special parts called active sites. These are like tiny pockets or shapes where the catalyst and the molecules that are reacting fit together perfectly, like puzzle pieces. When the molecules fit into the active sites, the reaction happens, and the molecules are transformed into something new. The active site makes the reaction faster and easier, without running out, so it can keep helping over and over (Figure 3A).
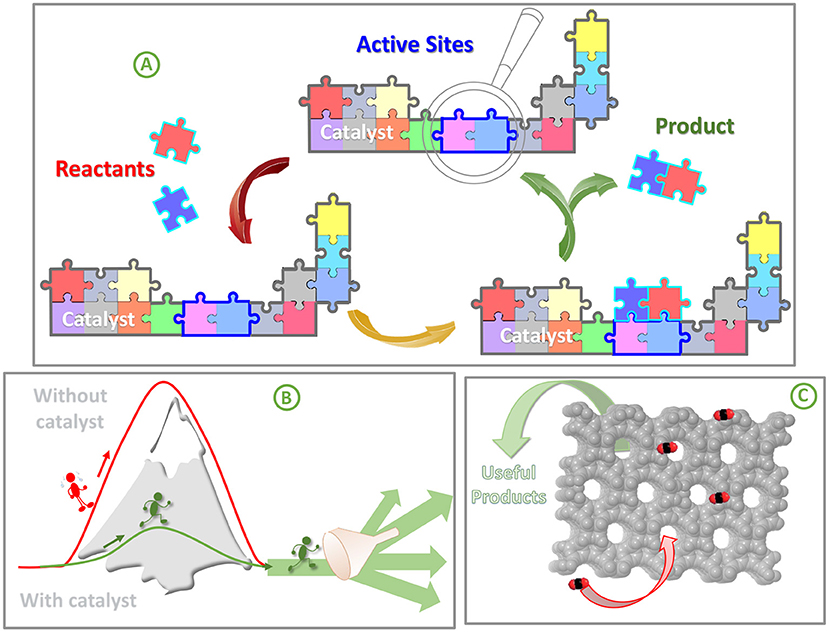
- Figure 3 - (A) Catalysts have active sites, which are special parts where the catalyst and the molecules to be reacted fit together perfectly like puzzles pieces.
- (B) Catalysts not only make reactions easier and speed them up but also direct them along selective pathway to obtain the desired products. (C) Catalysts like zeolite and OMS have tiny pores and channels that act like a molecular sieve and can choose which molecules to trap and react based on their molecular size, and drive the reaction toward the preferred products.
Good catalysts do not just speed up reactions—they also direct the reactions to make the “right” products and avoid unwanted production of unnecessary by-products (Figure 3B). This property, called selectivity, is key to making chemical processes more efficient and eco-friendly.
Catalysts can have different forms and state, can therefore be solids or liquids. Solid catalysts are easier to work with because they can be easily separated from liquid mixtures by filtration, the same way we separate pasta from water using a strainer. By making the transformation of molecules like CO2 more efficient, catalysts save energy, reduce costs, and help lower CO2 emissions.
Catalysts in Daily Life, Science, and Industry
Catalysts are all around us. For example, we have natural catalysts called enzymes in our bodies, which act as natural catalysts, helping to break down food into nutrients. Similarly, the yeast used in baking bread and biscuits acts as a catalyst to create the tiny bubbles that help the dough rise and become fluffy.
Scientists have made great discoveries in recent decades and have learnt how catalysts work. They explored the influence of catalysts on reaction conditions (e.g., heat, time, and ingredients) and rate. Moreover, the advances in technology and devices allow to study and monitor reactions at incredibly small scales, down to the level of molecules (microscope), nanostructures (1000 times smaller) or even single atom [1].
Catalysts are powerful tools that help chemists overcome the challenges of transforming molecules such as CO2 into useful products—all while saving energy and sources, reducing CO2 emissions, and protecting the planet [2]. Today, catalysts play a crucial role in producing over 80% of products we use, making them vital for industry and the economy. In the future, catalysts will be even more important for creating clean water, renewable energy, sustainable food supplies, and eco-friendly products.
But catalysts are not just tools for chemists, they are amazing tools for building a better world.
Zeolites and OMS: Powerful Catalysts
Zeolites and ordered mesoporous silica (OMS) are special materials mostly made of silica, a substance similar to beach sand. What makes these materials unique is their amazing structure. They are like super tiny sponges, full of microscopic pores and channels that are measured in nanometers (one billionth of a meter) (Figure 3C). Because of these tiny openings, zeolites and OMS are also called “molecular sieves” because they can sort molecules by controlling which can pass through them or get trapped inside, based on the size of the molecules. It is like trying to pass balls of different sizes through a sieve: the smaller ones go through, but the bigger ones do not. In this way, these catalysts can choose which molecules to trap and react, to drive the reaction toward preferred products according to their molecular size. Zeolites and OMS can have different shapes, types, and a huge variety of active sites. These adjustable characteristics allow scientists to customize their powers.
For over 100 years, zeolites and OMS have been used in industries for all sorts of tasks. They have helped make gasoline, clean water, and purified air. For the past 25 years, we have prepared powerful catalysts from these materials in our laboratory and used them for the eco-friendly manufacture of useful products, thus helping to protect the planet. These incredible materials may be tiny, but they are making a big difference in solving some of the world’s most difficult challenges.
Zeolite and OMS Can Convert CO2 Into Useful Products
In our laboratory, we have a lot of experience in the design and preparation of powerful catalysts from OMS and zeolites. We work on improving the performance of zeolite and OMS by creating powerful and sophisticated active sites for challenging transformations. These catalysts help us to transform CO2 into useful things, like methane, a fuel that can power homes and vehicles [3]. We also explore the possibility of preparing other valuable products. But in this quest, we must be careful that CO2 conversion does not use too much energy or too many raw materials, or the reactions can produce more CO2 than they save! As our work advances, powerful zeolites and OMS will be game-changers in the mission to recycle CO2 and turn it into something valuable.
In conclusion, zeolites and OMS are powerful tools that can be specifically designed to help us turn CO2 from waste into useful products and energy. These catalysts are helping us find exciting new ways to reduce CO2 emissions. However, no single solution is enough. We must combine efforts like capturing, reusing, and most importantly producing less CO2 to protect our planet and create a better future!
Glossary
Net Zero: ↑ Means to balance a scale. If we add something, we have to subtract the same amount to maintain the value, i.e., the balance is zero.
Decarbonization: ↑ Means stopping the increase in the amount of carbon in the air.
Sequestration: ↑ Means catching and keeping in storage.
Catalyst: ↑ Is a special substance that accelerates chemical reactions without being used up. Catalysts encourage molecules to lock together and form new products by reducing the efforts needed for the reaction.
Active Sites: ↑ Are like little shapes in which the catalyst and the molecules fit together perfectly. This is when the reaction takes place and the molecules are transformed into something new.
Selectivity: ↑ Catalysts not only accelerate reactions, but also direct them to obtain the right products and avoid the production of unnecessary by-products. This property is called selectivity.
Zeolite: ↑ Are tiny crystals called molecular sieves because of their pores and channels of microporous dimensions that act like a sieve, selecting which molecules to trap and react depending on their size.
Ordered Mesoporous Silica: ↑ Are tiny crystals called molecular sieves because of their pores and channels of mesoporous dimensions that act like a sieve, selecting which molecules to trap and react depending on their size.
Conflict of Interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
AV thanks Pablo A. Ramos (Instituto de Tecnología Química - Universitat Politècnica de València, Agencia Estatal Consejo Superior de Investigaciones Científicas, Valencia, Spain) for creating the Figure 1.
Original Source Article
↑Velty, A., and Corma, A. 2023. Advanced zeolite and ordered mesoporous silica-based catalysts for the conversion of CO2 to chemicals and fuels. Chem. Soc. Rev. 52:1773–946. doi: 10.1039/D2CS00456A
References
[1] ↑ Corma, A. 2016. Heterogeneous catalysis: understanding for designing, and designing for applications. Angewandte Chem. Int. 55:6112–6113. doi: 10.1002/anie.201601231
[2] ↑ Hutchings, G.J., and Haruta, M., 2005. A golden age of catalysis: a perspective. Appl. Catal. A Gen. 291:2–5. doi: 10.1016/J.APCATA.2005.05.044
[3] ↑ Velty, A., and Corma, A. 2023. Advanced zeolite and ordered mesoporous silica-based catalysts for the conversion of CO2 to chemicals and fuels. Chem. Soc. Rev. 52:1773–946. doi: 10.1039/D2CS00456A
