Abstract
The search for life on Mars has fascinated humanity for a long time. Scientists study the red planet using telescopes on Earth, spacecrafts that fly around it, and robots that have landed on its surface. Evidence indicates that Mars has underground liquid water, a critical resource for all living beings on Earth. There is also evidence of some interesting molecules formed by the perchlorate anion () in the Martian soil. On Earth, these molecules can be used by certain types of microbes to grow. This means that microbes capable of using perchlorate salts as part of their life cycle could survive on Mars. In this article we discuss how Earth microbes use the perchlorate anion based on some lab experiments performed by our research group. Our work could eventually help to detect microbial activity on Mars.
What is the Perchlorate Anion?
As you may know, molecules are formed by the combination of atoms. The atoms in a molecule can be the same, like in an oxygen molecule with two oxygen atoms (O2); or the atoms can be different, like in a water molecule with one oxygen atom and two hydrogen atoms (H2O). Molecules can have no electrical charge (called neutral) or be positively charged (called cations) or negatively charged (called anions). The perchlorate anion is a unique arrangement of five atoms: one chlorine atom (Cl) surrounded by four equally spaced oxygen atoms (O). This group of atoms has a negative charge and is written as . Because it has a negative charge, it needs to join with a positively charged atom, like sodium (Na+), potassium (K+), or magnesium (Mg2+), to make a neutral molecule or a salt. In natural environments, salts like sodium perchlorate (NaClO4) or magnesium perchlorate [Mg(ClO4)2] can be found in soil and water, especially in dry places in the world like deserts. Also, some perchlorate salts can be produced as byproducts from the manufacture of matches, explosives, fertilizers, and military weapons and ammunition. When animals or plants are exposed to high quantities of perchlorate salts, the perchlorate anion tends to accumulate in their tissues, causing negative effects on their health. But some kinds of microbes can transform the perchlorate anion into a new, useful compound that does not harm them. By doing so these microbes, called perchlorate reducers, generate the energy they need to grow and stay alive [1, 2].
Microbes and Perchlorates
Microbes are among the smallest forms of life on Earth. We cannot see them with the naked eye, we need the help of a microscope. Up to now, perchlorate-reducing activity has only been reported in two main types of microbes: bacteria and archaea. Those perchlorate reducers naturally occur in water and soil rich in perchlorate molecules. They transform the perchlorate anion () into the chlorate anion () and finally into the chlorite anion (), in a process known as reduction (Figure 1). As you can see, the reduction of perchlorate to the chlorite anion means a decrease in the number of oxygen atoms bound to the Cl atom. The enzymes used in this process are called perchlorate reductase, chlorate reductase, and chlorite dismutase. These chemical transformations help the perchlorate-reducing microbes to obtain energy to grow [2]. In other words, just as humans use oxygen to breathe, these microbes use perchlorate to carry out their “breathing”.
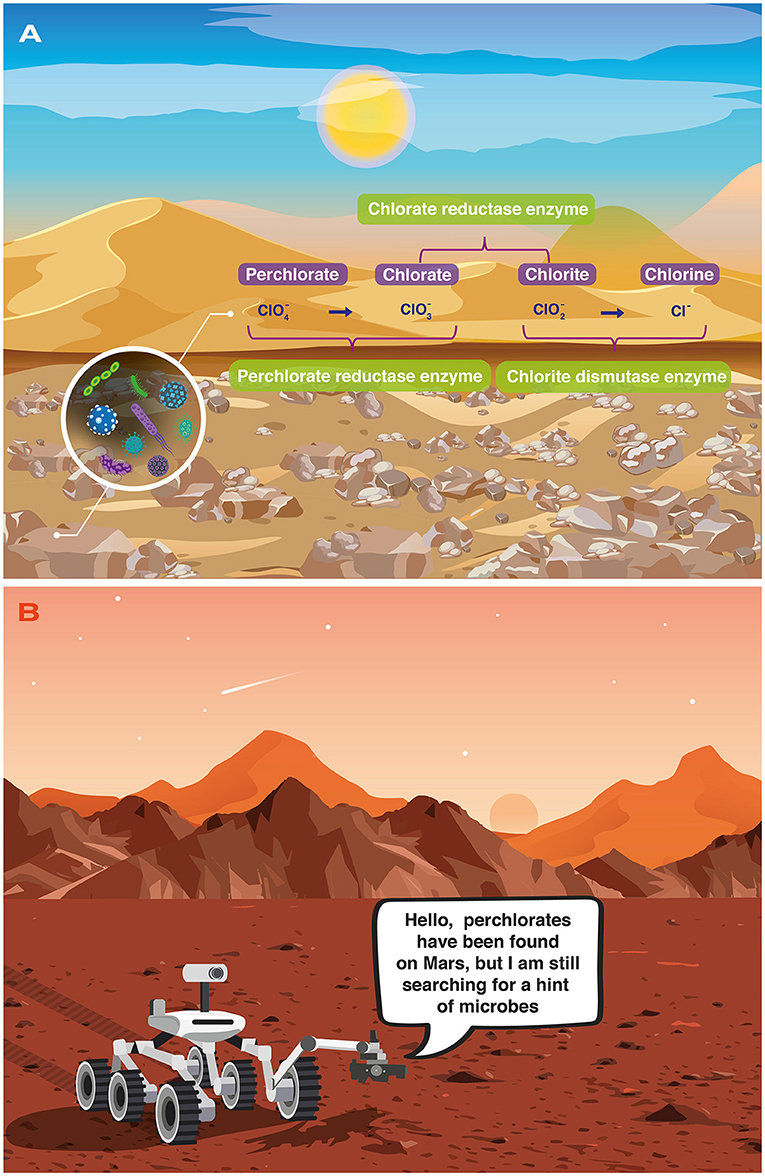
- Figure 1 - (A) The Atacama Desert on Earth is like the Mars environment because the perchlorate anion has been discovered in both places.
- In the Atacama Desert, microbes can break down the perchlorate anion with the help of specific enzymes. (B) The Perseverance rover exploring Mars and searching for perchlorate-reducer microbes.
Perchlorates in the Atacama Desert
On Earth, perchlorate salts are principally found in arid regions such as deserts. The Atacama Desert in South America is known as the oldest and driest desert in the world, receives a lot of sunlight, and has temperatures that can change from a chilly −3°C at night to a hot 50°C during the day. Scientists have discovered a lot of perchlorate salts in this desert. Because of these extreme conditions, the Atacama Desert is similar to the current surface of Mars. Scientists study this desert to learn about whether life could exist on Mars [3].
In the Atacama Desert, the hot sun and the strong winds make water disappear very quickly, leaving behind special rocks that are rich in salts called evaporites. What is really cool about evaporites is that tiny microbes can actually live inside them! These microbes are called halophiles, which means “those who love salty environments”. You probably know that the most common salt on Earth is sodium chloride (NaCl). We used NaCl to season our food, but it is only one example of a wider group of substances. In chemistry, a molecule of salt is made when two kinds of particles stick together: one cation and one anion. In the Atacama Desert and on Mars, sodium perchlorate (NaClO4) and magnesium perchlorate [Mg(ClO4)2] are examples of salts [3].
Perchlorates On Mars
Mars is the fourth planet in the solar system and the closest to Earth. It is only about one-tenth the size of Earth. Mars is a cold place, with an average temperature of about −60°C. Its atmosphere is very thin and mostly made of carbon dioxide (CO2). Mars also has ice caps, just like Earth, and there is even some water trapped underground [4].
Several Mars missions have identified Cl in the Martian soil. In 1976, a spacecraft called the Viking lander found more Cl on Mars than the quantity identified by scientists in the Earth’s soils. At first, they thought the Cl might be in the same form as on Earth, like NaCl, but a spacecraft named Odyssey, which flew over Mars in 2001, found evidence suggesting that the chlorine atom was part of the perchlorate anion (). In 2008, the Phoenix mission discovered small amounts of the perchlorate anion, about 0.5%-1.0% of the soil, in northern regions of Mars, particularly in a place called the Gale Crater. The Curiosity rover also found perchlorate in the southern part of Mars. Scientists have even discovered the perchlorate anion in a Martian meteorite, a rock from Mars called EETA79001, when they studied it in laboratories on Earth [5].
Studying Microbes in Simulated Martian Conditions
Studying the way that perchlorate-reducing microorganisms handle perchlorate in tough environments helps scientists to predict if these microbes could survive in places different from Earth. In laboratories, scientists can create conditions that mimic those extreme environments and then use some specific microbes that are known to grow in perchlorate levels similar to the ones found on the surface of Mars (Figure 2). This type of research can help to demonstrate that life might be able to exist in harsh places like Mars!

- Figure 2 - Scientists on Earth can set up special experiments in labs to mimic real Martian conditions.
- They do this to see if microorganisms from Earth can survive in conditions similar to those existing on Mars.
Our research group is interested in the effect that different amounts of two perchlorate salts, NaClO4 and Mg(ClO4)2, have on the growth of certain halophilic bacteria. We already know that these bacteria can grow at perchlorate levels similar to those found on the surface of Mars. This information is exciting because it helps us to understand how terrestrial bacteria might adapt to Martian conditions, specifically with respect to the presence of perchlorates. However, understanding Mars is not just about studying perchlorate levels—the Martian environment is much more complicated. We try to mimic real Martian conditions more closely in our experiments by applying low temperatures, using simulated Martian soil, and creating atmospheres rich in CO2–the main atmospheric gas on Mars. It is important to note that, so far, there is no scientific proof that microbes exist on Mars [6, 7], but we believe our experiments can help predict the chances of finding life beyond Earth!
What We Learned
Mars is a super interesting planet for scientists to study. It is very cold and dry, has a thin atmosphere, lots of harmful ultraviolet rays, and some particular chemicals called perchlorates. These characteristics make Mars a harsh place for life as we know it. Scientists use special instruments to recreate Martian conditions to evaluate if certain microbes might survive in those conditions. This helps us understand whether the same type of life forms that live on Earth could also survive on other planets—a key aspect of planning future space missions to Mars.
Glossary
Cation: ↑ A group of atoms with a positive net charge due to the loss of at least one electron.
Anion: ↑ A group of atoms with a negative net charge due to the gain of at least one electron.
Perchlorate: ↑ A chemical compound that consists of a chlorine atom surrounded by four equally spaced oxygen atoms. It is negatively charged and needs a cation to form a salt.
Archaea: ↑ One of the three domains of life that usually includes microbes found in extreme environments.
Reduction: ↑ The gaining of at least one electron by an atom that participates in a chemical reaction.
Enzyme: ↑ A macromolecule that helps to increase the reaction rate in specific chemical transformations.
Evaporites: ↑ A layered sedimentary rock formed from brines when water is lost by evaporation.
Halophile: ↑ An extreme organism that thrive in environments with high salt concentrations.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by a grant from CONAHCYT (A3-S-65162). Santiago Cadena thanks to CONAHCYT for the postdoctoral grant 570049-2024-2025. We thank Anabel Suárez Guevara for the figures design, and Susan Debad for her deep involvement in the edition of the manuscript.
References
[1] ↑ Nerenberg, R. 2013. Breathing perchlorate. Science 340:38–9. doi: 10.1126/science.1236336
[2] ↑ Barnum, T. P., Figueroa, I. A., Carlström, C. I., Lucas, L. N., Engelbrektson, A. L., and Coates, J. D. 2018. Genome-resolved metagenomics identifies genetic mobility, metabolic interactions, and unexpected diversity in perchlorate-reducing communities. ISME J. 12:1568–81. doi: 10.1038/s41396-018-0081-5
[3] ↑ Cadena, S., Cerqueda-García, D., Uribe-Flores, M. M., and Ramírez, S. I. 2024. Metagenomic profiling of halites from the Atacama Desert: an extreme environment with natural perchlorate does not promote high diversity of perchlorate reducing microorganisms. Extremophiles 28:25. doi: 10.1007/s00792-024-01342-6
[4] ↑ Wright, V., Morzfeld, M., and Manga, M. 2024. Liquid water in the Martian mid-crust. PNAS. 121:e2409983121. doi: 10.1073/pnas.2409983121
[5] ↑ Carrier, B. L., and Kounaves, S. P. 2015. The origins of perchlorate in the Martian soil. Geophys. Res. Lett. 42:3739–45. doi: 10.1002/2015GL064290
[6] ↑ Avendaño, R. E., Montoya, L., Olmos, J., and Ramírez, S. I. 2015. Growth of Bacillus pumilus and Halomonas halodurans in sulfates: prospects for life on Europa. Bol. Soc. Geol. Mex. 67:367–75.
[7] ↑ Oren, A. 2014. Halophilic archaea on Earth and in space: growth and survival under extreme conditions. Philos. Trans. R. Soc. A. 372:20140194. doi: 10.1098/rsta.2014.0194
