Abstract
As humans, we can use words like “hungry” and “full” to communicate when we need to eat throughout the day. However, mice, which are commonly used to study feeding behaviors in the laboratory, cannot tell us what they are feeling. We trained mice to tell us whether they were hungry or full. Then, we turned on and turned off certain cells in a brain region called the hypothalamus to see if these specific cell types could make a mouse feel hungry or full. Our research showed that turning on specific brain cells in a region called the arcuate nucleus of the hypothalamus caused mice to report they were hungry, even if they had just eaten and their stomachs should feel full. These results give us clues about how the brain works to control hunger.
Why Do We Eat?
Most of us have experienced hunger at some point in our lives—that nagging, unpleasant feeling in your stomach after skipping a meal or exercising. You feel this way because your gut is sending hormone signals to the brain saying that your body needs more nutrients and energy. To eliminate this feeling, you may reach for a snack or eat a meal. Then, your senses and more hormones from the gut signal back to the brain when it is time to stop eating, and you feel full. This is called homeostatic eating—eating because your body needs more energy to continue to function properly.
You probably agree that this is a different reason to eat than, for example, seeing your favorite dessert in front of you after dinner. You may not feel hungry after dinner, but you might eat the dessert anyway because you know it will taste good. This is called hedonic eating—eating because it just tastes good or makes you feel good, not because of hunger. This illustrates that there are different reasons why humans eat. In our research project, we aimed to understand how the brain controls hunger.
Feeding and the Brain
The hypothalamus is a brain region required for regulating many important functions, like eating, sleeping, and body temperature. We study brain regions and cells that regulate eating. Different populations of neurons in the hypothalamus have been identified based on the genes they express; we call these genetically identified neurons. Further, we can categorize neurons by the specific region of the hypothalamus where they are found. Knowing the region and the genetic identity of the neurons, we can specifically turn those cells on (activate them) or turn them off (inhibit them) (to learn more about the technique used to do this, read this Frontiers for Young Minds article). We can do this because we work with mice instead of humans. A mouse’s neurons can be modified using special viruses that do not harm animals. By manipulating genetically identified neurons in this way, we can learn how specific neurons and brain regions influence feeding behaviors.
Many neuron populations in the hypothalamus are important for feeding. We will focus on three of these populations (Figure 1A). The first is in the arcuate nucleus of the hypothalamus (ARC), which expresses a gene called Agrp. We call these ARCAGRP neurons. In a different sub-region called the lateral hypothalamus (LH), there are two groups of neurons that express genes called Vgat or Vglut2. We call these LHVGAT and LHVGLUT2 neurons. Scientists have shown that activating or inhibiting these three cell populations can make mice eat or not eat. Specifically, activating ARCAGRP and LHVGAT neurons in sated (full) mice will make them eat much more than normal even though they should not be hungry (Figure 1B) [1–3]. Inhibiting these neurons when mice are fasted (hungry) makes them eat much less than normal even though they should be hungry [2, 3]. Interestingly, LHVGLUT2 neurons do the opposite. Activating LHVGLUT2 neurons reduces the amount of food that fasted mice eat, while inhibiting LHVGLUT2 neurons increases the amount of food that sated mice eat [4].
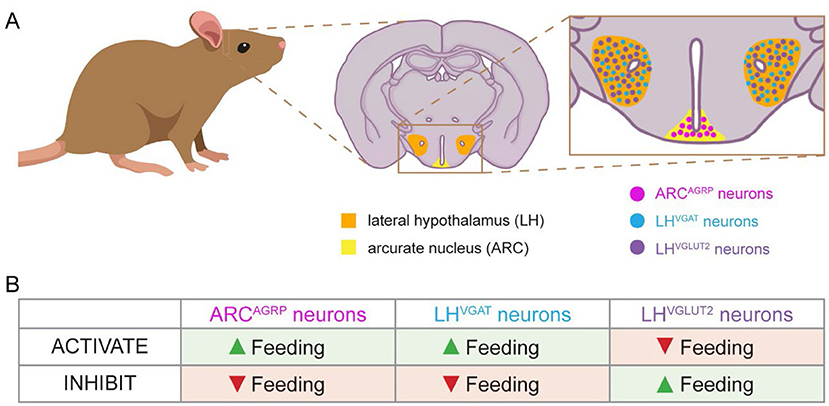
- Figure 1 - Genetically identified neurons in the hypothalamus are involved in feeding behaviors.
- (A) The mouse brain has regions called the lateral hypothalamus (orange) and the arcuate nucleus of the hypothalamus (yellow). These regions contain genetically identified neurons known to regulate feeding behavior. We studied ARCAGRP, LHVGAT, and LHVGLUT2 neurons. (B) This chart shows how these neuron populations affect feeding behavior. When a neuron is activated, it sends messages to other cells. When a neuron is inhibited, it stops communicating with other cells. Neurons turn on and off as needed to regulate feeding behavior.
Sated vs. Fasted: Designing a Feeding Experiment
You may wonder why we work with mice that are either sated or fasted. We do this to maximize the effects we see when activating or inhibiting neurons. If a mouse is fasted and it eats, we do not know if feeding was caused by our manipulation or because the mouse was just hungry. Similarly, if a mouse is sated and it does not eat, we cannot determine whether it was our manipulation or if the mouse simply was not hungry. However, if we can make a fasted mouse continue to deprive itself of food, or make a sated mouse eat even more food, we can then say with more certainty that our manipulations were responsible for those effects. So, to test whether neurons make a mouse eat more food, we start with a sated mouse. To test whether neurons make a mouse eat less food, we make sure the mouse is fasted first.
Can a Mouse Tell Us If it is Hungry?
As we discussed earlier, there are different reasons why humans (and mice!) eat. We should not assume that an animal is hungry because it is eating, or that an animal is not eating because it is full. How can a mouse tell us whether it feels hungry or full?
To address that question, we first trained mice to press one lever when they were fasted and a different lever when they felt sated (Figures 2A, B) [5]. Like humans, mice also enjoy a sweet treat. So, we gave mice sugar pellets as a reward for pressing the correct lever. Mice learned to press the “sated” lever after food was removed for 1 h. Mice learned to press the “fasted” lever when we removed food for 22 h. We reasoned that preventing the mice from eating for 22 h (almost 1 day!) would ensure they were hungry. This would feel much different to the mice than only 1 h without food, when they were unlikely to feel hunger.
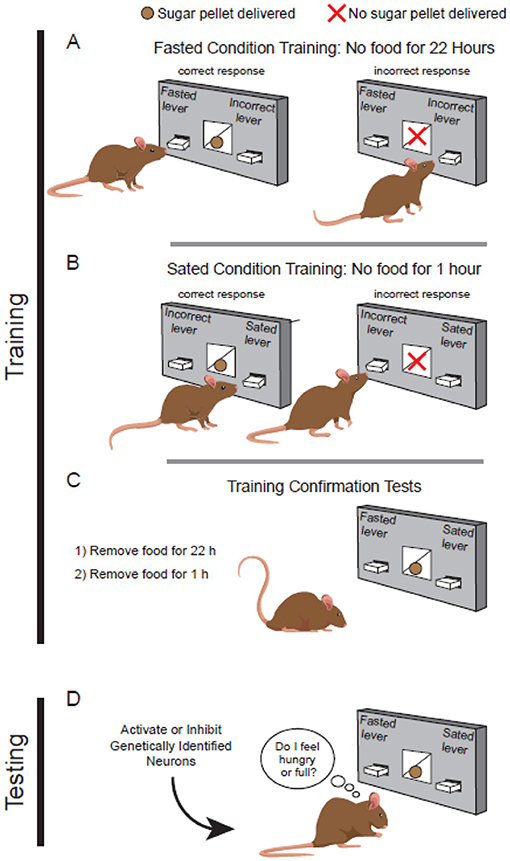
- Figure 2 - Mice discriminate between hunger and satiety.
- (A) Fasted mice feel hungry, and they learn to press a specific lever to receive a sugary reward. (B) Mice without food for only 1 h are not hungry, but they learn to press the “sated” lever to receive a sugar pellet. (C) After training, fasted or sated mice can decide which lever to press based on how they feel. Trained mice can tell the difference between hunger and satiety. (D) Once mice are trained, we can manipulate genetically identified neurons to see if the manipulation makes mice feel hungry or full.
After many weeks of training, mice could reliably press the correct lever for both fasted and sated conditions. Then we tested them to confirm they were fully trained. During the test, mice received a sugar reward no matter which lever they pressed (Figure 2C). We tested mice once after the fasted condition and once after the sated condition. Doing this test under both feeding conditions allowed us to make sure the mice could tell the difference between how they felt—either hungry or full—without relying on just the sugar reward to guide them to the correct lever. In other words, we knew mice were trained when they could finally accurately report whether they were hungry or not.
Can Neuron Manipulations Make Mice Feel Hungry?
Previous experiments showed how activating or inhibiting different types of genetically identified neurons affects feeding behavior (Figure 1B). With our trained mice, we could determine whether any of those activations or inhibitions caused mice to feel hunger or satiety. Before testing, we determined the feeding conditions (sated vs. fasted) required to test each type of genetically identified neurons. Then, we tested our trained mice while activating or inhibiting each neuron population (Figure 2D).
We found that under a fasted state, inhibition of ARCAGRP neurons did not cause mice to press the sated lever, meaning inhibition had no effect on the mice’s feeling of hunger. However, activation of LHVGLUT2 neurons and inhibition of LHVGAT neurons did cause fasted mice to press the sated lever (Figure 3). In other words, even though the mice had not eaten for almost a day, they reported feeling full! So, these two types of neurons can cause a mouse to feel sated, but what about causing hunger?
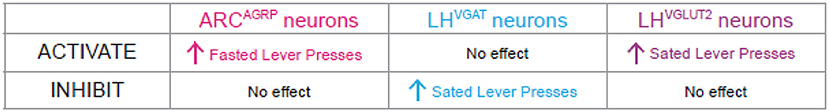
- Figure 3 - Activation of ARCAGRP neurons makes mice feel hungry.
- We tested three different genetically identified neuron populations. Our results indicated that LHVGAT and LHVGLUT2 neurons might contribute to satiety. Additionally, LHVGAT neurons may be involved in hedonic eating. Only activation of ARCAGRP neurons made mice press the fasted lever a lot, even though the mice were sated. This means that ARCAGRP neurons are hunger neurons involved in homeostatic eating. When active, these neurons contribute to our feeling of hunger, telling us that our bodies need food for nutrients and energy.
We found that, under sated conditions, inhibition of LHVGLUT2 neurons did not make mice press the fasted lever, which means they still felt full. Interestingly, activation of LHVGAT neurons did not cause the sated mice to press the fasted lever, even though it caused them to eat more. We concluded that, although activation of LHVGAT neurons caused mice to eat more, they were not eating due to hunger—that is, it was not homeostatic eating. LHVGAT neurons may be involved in hedonic eating instead. Finally, activation of ARCAGRP neurons caused sated mice to press the fasted lever. Therefore, activating ARCAGRP neurons made the mice respond as if they were hungry even though they were sated. We can now say that ARCAGRP neurons are hunger neurons.
Why is This Important?
Many of us do not think about the brain when we talk about hunger. We are far more likely to associate hunger with the stomach because that is where we feel it. However, it is important to remember that your brain works with the rest your body to coordinate everything you do and feel. The different populations of neurons described in this article (along with many other neuron populations that we did not discuss) regulate when we need to eat and when we should stop eating. Sometimes diseases and mental illnesses alter normal eating habits and brain function. Eating disorders are very serious mental illnesses that can lead to extreme food restriction, binge eating, or extreme overeating. Furthermore, obesity is recognized worldwide as a major health concern because it can lead to numerous health conditions including diabetes, high blood pressure, and heart disease. Understanding how the brain regulates feeding behaviors will help us understand how the brain may function differently during disease and mental illness. Hopefully one day this knowledge will help scientists and doctors develop treatments for eating disorders and obesity.
Glossary
Hormones: ↑ Chemicals that travel in the blood to send messages coordinating body functions.
Homeostatic Eating: ↑ Eating because we are hungry, and the body needs energy to function properly.
Hedonic Eating: ↑ Eating because the food makes us feel good or tastes good, not because we are hungry.
Hypothalamus: ↑ A brain region that controls many important functions such as hunger, thirst, sleep, and body temperature.
Neurons: ↑ Cells of the nervous system that communicate with other cells using chemical or electrical signals. Neurons work together to coordinate bodily functions and behaviors including movement, hunger, vision, and memory.
Genetically Identified Neurons: ↑ Nerve cells that are identified by the genes they express.
Sated: ↑ The condition of feeling full after eating enough food to satisfy the body’s needs.
Fasted: ↑ The condition of being without food for a long period of time. Animals (including humans) usually feel hungry if they go without food for too long.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors acknowledge with gratitude members of the Aponte Lab and H. Sarsfield (age 14) for discussions and comments on the manuscript. Figures were created in collaboration with NIDA IRP Visual Media (D. Taylor). This work was supported by the National Institute on Drug Abuse Intramural Research Program (NIDA IRP) (ZIADA000595), U.S. National Institutes of Health (NIH). YP-H was supported in part by the Solomon H. Snyder Department of Neuroscience, Johns Hopkins University School of Medicine.
Original Source Article
↑Siemian, J. N., Arenivar, M. A., Sarsfield, S., and Aponte, Y. 2021. Hypothalamic control of interoceptive hunger. Curr. Biol. 31:3797–3809.e5. doi: 10.1016/j.cub.2021.06.048
References
[1] ↑ Aponte, Y., Atasoy, D., and Sternson, S. M. 2011. Agrp neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nat. Neurosci. 14:351–5. doi: 10.1038/nn.2739
[2] ↑ Krashes, M. J., Koda, S., Ye, C., Rogan, S. C., Adams, A. C., Cusher, D. S., et al. Rapid, reversible activation of agrp neurons drives feeding behavior in mice. J. Clin. Invest. (2011) 121:1424–8. doi: 10.1172/JCI46229
[3] ↑ Jennings, J. H., Rizzi, G., Stamatakis, A. M., Ung, R. L., and Stuber, G. D. The inhibitory circuit architecture of the lateral hypothalamus orchestrates feeding. Science (2013) 341:1517–21. doi: 10.1126/science.1241812
[4] ↑ Stamatakis, A. M., Van Swieten, M., Basiri, M. L., Blair, G. A., Kantak, P., and Stuber, G. D. Lateral hypothalamic area glutamatergic neurons and their projections to the lateral habenula regulate feeding and reward. J. Neurosci. (2016) 36:302–11. doi: 10.1523/JNEUROSCI.1202-15.2016
[5] ↑ Siemian, J. N., Arenivar, M. A., Sarsfield, S., and Aponte, Y. Hypothalamic control of interoceptive hunger. Curr. Biol. (2021) 31:3797–3809.e5. doi: 10.1016/j.cub.2021.06.048