Abstract
Water is needed for almost every aspect of human life: cleaning, drinking, and crop production. Membrane filtration is an effective way to remove the pollutants from water. Tiny holes in the membranes allow water to pass through while trapping dirt, bacteria, and other pollutants. Most water-filtration membranes can remove large pollutants such as hair, dust particles, and bacteria, as well as pollutants that cannot be seen with the naked eye, such as dissolved salts. In this article, we will tell you how membranes are made and how they can be used to make water clean and safe enough for people to drink.
The World Needs Clean Water!
Of the total amount of water available throughout the world, only 3% is safe for human consumption. This water is available in the form of surface water (rivers and lakes) and underground water (wells and springs). The remaining 97% is in the oceans and not suitable for humans. Available fresh water has decreased over time due to the increase in populations and industries (especially pharmaceuticals, manufacturing, mining, and agriculture). The reduced availability of good-quality fresh water means that the world needs to study and develop advanced water-treatment technologies to enable the safe reuse of wastewater. Wastewater refers to water that has already been used and therefore contains harmful impurities called pollutants—for example, water from your kitchen or laundry.
Drinking water polluted with microorganisms can result in diseases such as cholera, diarrhea, and many more. With the increases in both human populations and the use of medications, new kinds of pollutants that are difficult to remove or break down into less toxic substances have become a problem in most wastewater treatment facilities. This has led to concerns about the consumption of these substances and what our digestive systems produce after interacting with them, since their health effects are often unknown [1]. As a result, research is being conducted to develop better wastewater treatment systems, so that people can have access to safe, treated water.
Membrane filtration is a method used to remove pollutants such as the salts in ocean water, dyes in wastewater from the textile industry, dirt from ground and surface water, and many more. The effectiveness of membrane filtration allows for water recycling, which can help reduce water shortages. Recycling water is good for the environment and helps to preserve the small percentage of Earth’s available water.
Membrane Filtration: Removing Dirt From Water
A membrane is a barrier with tiny holes called pores that are not visible to the naked eye but can be seen under a microscope. The pores are mostly only nanometres in diameter (a nanometre is one billionth of a meter), and they allow water to pass through while preventing the passage of pollutants. You can think of membrane filtration like sifting something through a mesh or a screen. The mesh keeps large particles on one side while letting small ones through. In membrane filtration, the membranes are classified into classes with respect to their pore sizes: microfiltration (MF), ultrafiltration (UF), nanofiltration (NF), and reverse osmosis (RO) [2]. The pore sizes get smaller in order from MF through RO. Membranes can remove pollutants ranging from those that can be seen with the naked eye (like dust particles and lint) to nano-sized particles (bacteria and proteins) and dissolved salt ions that cannot be seen (Figure 1). The amount of pressure that is used to drive water through the pores is another condition that makes filtration membrane types different from each other.
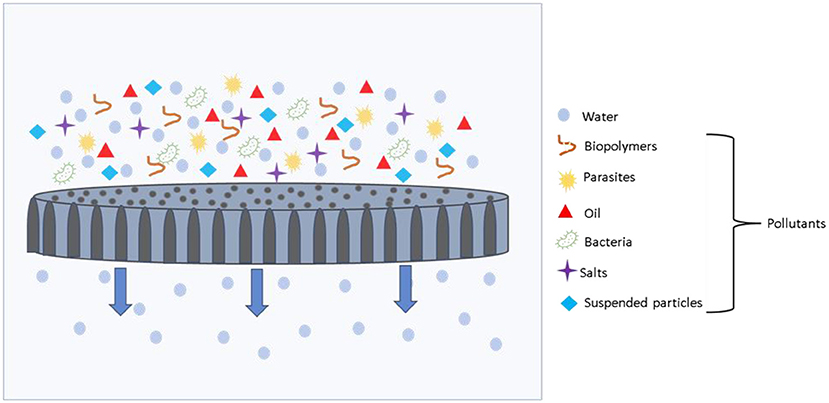
- Figure 1 - A membrane sheet sifting out unwanted pollutants (various colored shapes) from water during the filtration process.
- Membranes can remove all sizes of pollutants, from large pollutants like particles of dirt and parasites, all the way down to tiny salt molecules. The water can move through the holes in the membrane and come out clean on the other side.
Fouling: When Membranes Get Clogged
Due to size variation, some pollutants can fit through the pores of the membranes and attach to the pore walls, building up there and blocking the pores. Fouling, defined as the blockage of the membrane pores by the pollutants in the water, is of major concern in membrane filtration. Blockage of pores slows down and ultimately prevents water from passing through the membranes. To minimize the accumulation of pollutants on the surface of the membranes and within their pores, a technique called back-washing is used. During back-washing, high-pressure clean water is run in the opposite direction to the filtration direction, pushing the pollutants off the membrane surface and out of the pores. Some pollutants, however, attach strongly to the membrane surface, forming a chemical linkage with the membrane [3]. In such cases, chemicals are used to break the linkages during back-washing. Membrane fouling can cause physical damage to the membrane, decreasing water quality and reducing the rate at which water passes through the membrane. This shortens the amount of time that membranes can be used, which leads to the need to frequently replace them.
One of the easiest ways to reduce membrane fouling is to start with another pollutant-removing method, called pre-treatment of water. Pre-treatment removes or reduces the pollutants that can cause fouling. One pre-treatment processes involves the addition of chemicals that cause the pollutants in water to stick together and form clumps that settle out of the water, which is called flocculation. Not only does pre-treatment prevent clogging, but it can also remove ~70% of the pollutants, even the smallest ones, which means the membrane only has to remove the remaining pollutants, thereby producing higher-quality water. Wastewater that is pre-treated has low fouling potential, so membranes can be used for a longer period.
How are Membranes Made?
Membranes are made of different kinds of materials: sand/clay particles for ceramic membranes, polymer materials for polymeric membranes, and specific metals, such as palladium, for metallic membranes. Recent developments involve mixing various membrane-making materials to make new membranes that are more effective or selective for removing just certain pollutants. These are called mixed-matrix membranes.
The process of making membranes is quite easy. The first step is to make a solution containing a polymer, a liquid called a solvent, and nanomaterials to control the properties and behavior of the membranes. This solution is often referred to as a dope solution (Figure 2A). Next, the dope solution is spread on a sheet of glass using a flat piece of metal called a casting knife (Figure 2B), and the dope-covered glass plate is then placed in a container of water (Figure 2C). After a few minutes, the dope solution changes to a solid form, creating a flat sheet of membrane (Figure 2D). Figure 3 shows how membranes appear under a microscope.
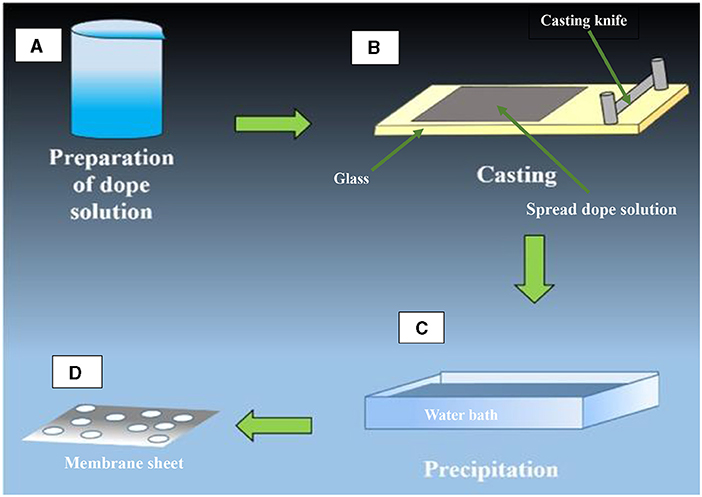
- Figure 2 - Steps in the preparation of a flat-sheet membrane.
- (A) A polymer powder is dissolved in a liquid (solvent) to make a dope solution. (B) The dope solution is spread on a sheet of glass using a solid, flat piece of metal called a casting knife. (C) The glass with the dope solution spread on it is then immersed in water bath, which causes the solution to change from a liquid state to a solid state. (D) The solid plastic-like membrane sheet with pores is then complete [4].
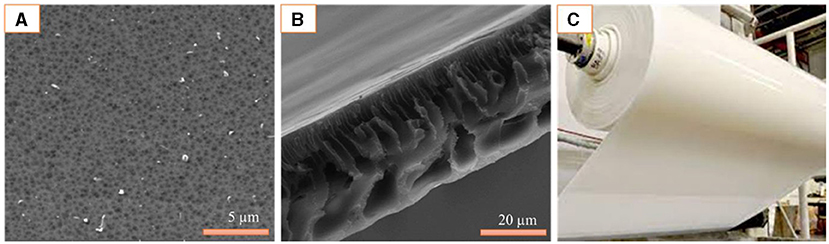
- Figure 3 - (A) A view of the top surface of a membrane through a microscope, with the pores appearing as dark, spot-like structures.
- (B) A microscopic cross-section or side-surface image, showing the empty tunnels of the membrane pores. (C) A roll of flat-sheet polymer membrane [5].
Summary
Different types of filtration materials are used to clean dirty water, producing water that is clean and safe to drink. This is important because reusing treated water can supplement available clean water suitable for safe use. Commonly used membranes are made from polymers because they are generally less expensive to produce and easier to handle than ceramic-type membranes. Combining filtration with pre-treatment processes generally results in longer membrane life and reduces the overall cost of wastewater treatment over the long term. Currently, membranes are the most efficient process in turning wastewater treatment into drinkable water, as well as for removing the salt from seawater.
Glossary
Pollutants: ↑ Substances or chemicals present in water making it not fit for its intended purpose.
Membrane Filtration: ↑ A process by which impurities are removed from a fluid, either a liquid or a gas, using a sheet with pores in it.
Fouling: ↑ Deposition of pollutants on the membrane surface and pores, hindering water passage.
Back-washing: ↑ Passing clean water or chemicals through the membrane in the opposite direction of water treatment, dislodging the deposited pollutants.
Pre-treatment: ↑ The process of removing or reducing pollutants in wastewater before cleaning it with another treatment system such as chemical flocculation.
Flocculation: ↑ The use of chemicals that are like magnets, to cause dirt and particles to stick together and form clumps that settle out of the water.
Polymers: ↑ Very large molecules made up of many smaller molecules called monomers, linked together in a repeating pattern. Plastic is an example of a polymer.
Mixed-matrix Membrane: ↑ Membranes made of organic polymer containing inorganic nanomaterials to control the membrane properties.
Nanomaterial: ↑ Substances with sizes between 5 and 100 nm in at least one dimension.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
[1] ↑ Lakshmi, V., Fayne, J., and Bolten, J. 2018. A comparative study of available water in the major river basins of the world. J. Hydrol. 567:510–32. doi: 10.1016/J.JHYDROL.2018.10.038
[2] ↑ Dalanta, F., Handoko, D. T., Hadiyanto, H., and Kusworo, T. D. 2024. Recent implementations of process intensification strategy in membrane-based technology: a review. Chem. Eng. Res. Des. 202:74–91. doi: 10.1016/J.CHERD.2023.12.014
[3] ↑ Wang, L., Li, Z., Fan, J., and Han, Z. 2024. The intelligent prediction of membrane fouling during membrane filtration by mathematical models and artificial intelligence models. Chemosphere 349:141031. doi: 10.1016/J.CHEMOSPHERE.2023.141031
[4] ↑ Remanan, S., Sharma, M., Bose, S., and Das, N. C. 2018. Recent advances in preparation of porous polymeric membranes by unique techniques and mitigation of fouling through surface modification. ChemistrySelect 3:609–33. doi: 10.1002/SLCT.201702503
[5] ↑ Matebese, F., and Moutloali, R. M. 2021. Greywater reclamation: a comparison of the treatment performance of UiO-66-NH2@GO nanocomposites membrane filtration with and without activated carbon pretreatment. J. Environ. Chem. Eng. 9:104906. doi: 10.1016/j.jece.2020.104906