Abstract
Bones are vital and strong, but not invincible—they can be broken. Normally, bones can repair themselves. However, sometimes broken bones must be supported using what is called an implant. An implant is a man-made device used to replace or support a missing part of the human body. In the case of a bone, an implant aims at helping the bone regain its natural function. However, inserting a foreign object into the body has several possible complications. In particular, bone-repair implants are often associated with difficult-to-fight bacterial infections. Many approaches and materials are being studied to improve bone implants. Our team based our bone implants on the bacteria-killing structures present on certain insects. Our results provide evidence that these natural structures could help us develop materials to improve bone repair, while helping to prevent bacterial infections.
Helping the Bone Repair Itself
Bone is an incredible tissue that changes dramatically throughout life. It continually breaks itself down and produces new bone, in a way that you cannot see or feel. Bones are primarily made of minerals, such as calcium and phosphate, but the key players are several types of cells that supply, organize, and shape the minerals. Bone-forming cells called osteoblasts give bone its ability to repair itself after a bone is broken (read more about it in this Frontier for Young Minds article). This repair process is called bone regeneration. Doctors put casts on broken limbs to immobilize them, so that the bone can slowly regenerate. However, in some situations, the defect (the hole created in the bone by the break) is too large to allow the recovery of full function. In this situation, doctors can use implants to fill the defect and help the body to repair the bone. However, inserting a foreign material into the body can lead to several complications.
The Challenge of Bacterial Infections
Bacteria are the most abundant form of life and are present almost everywhere on Earth. There are bacteria in the soil, water, air, and even in and on our bodies. These tiny organisms are essential to life on Earth and generally do not harm us. However, some types of bacteria tend to grow in tissues where they should not—this is what we call infection. In most cases, the body can defend itself against infection and return to normal. But in some cases, if the bacteria are aggressive or if the body is too weak to fight them efficiently, doctors must treat infections with drugs called antibiotics. If bacteria are present in the environment during implantation, they will multiply rapidly on the surface of the implant. This is a real problem for doctors because, although antibiotics are effective at killing bacteria, these drugs struggle to control bacteria growing on bone implants. In these cases, the implant often must be removed, which means more risk and pain for the patient.
In recent years, doctors and scientists have begun to realize that the more antibiotics are used, the more bacteria can adapt and find ways to survive in the presence of antibiotics (see this Frontiers for Young Minds article). Today, antibiotics are still incredibly useful, but we must start finding other ways to defend ourselves against bacteria.
Getting Inspired by Nature
In our search for a strategy to protect ourselves from bacteria, one approach is to mimic methods that already exist in nature. Researchers have observed such a feature on the wings of insects like the cicada. Very small pillars, several times smaller than bacteria, are present on the entire surface of the cicada’s wings (Figure 1). These sharp pillars can pierce and kill the bacteria that try to stick to the wings. This is a natural solution to the threat of bacterial infection. It is important to note that the size of these pillar structures is in the nanometer (nm) range, thus they are called nanostructures. One nanometer is one billionth of a meter. If you look at the size of a millimeter on a ruler, try to imagine that, in that tiny space, you could fit 1,000,000 nanometers.
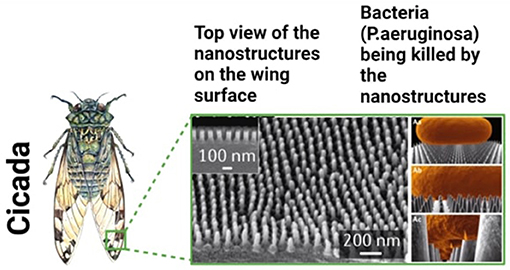
- Figure 1 - Nanostructures from an insect’s wings can kill bacteria.
- Zooming in on the wings of a cicada using a powerful microscope, we can see that the surface is not smooth—it has many small pillars. On the right you can see a bacterium (orange) trying to stick to the surface. The pillars on the surface of the cicada wings pierce the bacterium, leading to its death. (Figure adapted from [1] and created with BioRender.com).
Elena Ivanova is one of the researchers who saw great potential in mimicking these small structures to develop materials with antibiotic properties. For this purpose, she and her group created artificial pillars, similar to those found in nature, on the surface of various materials. They have developed two types of pillar-like structures that look like spikes or sharp edges, and which are very effective at killing bacteria (Figure 2).
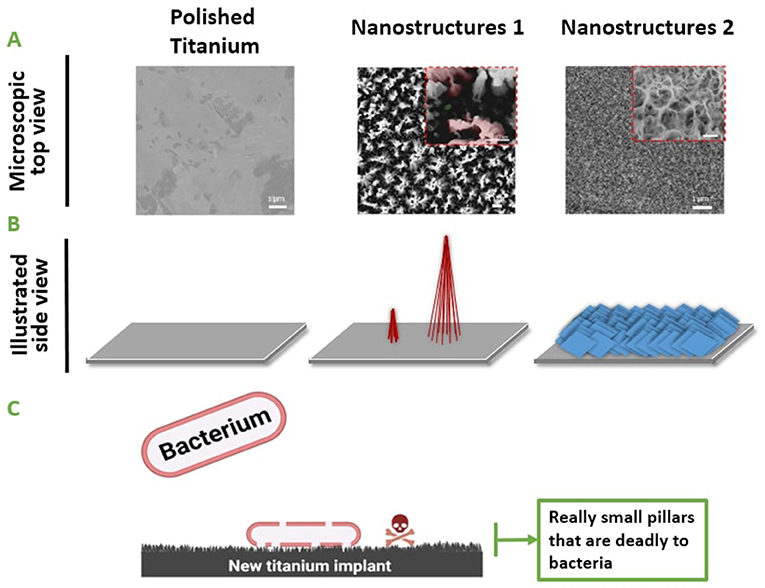
- Figure 2 - (A) If we use a microscope to zoom in on the surface of our engineered titanium, we can see the details of the nanostructures created by Ivanova’s team.
- On the left, you can see the smooth surface of titanium, and the two different types of nanostructured titanium are on the right. (B) Illustrations of the same surfaces shown in (A) (Figure adapted from [2]). (C) The engineered titanium surfaces were tested for their ability to kill bacteria. When we put bacteria on titanium that had nanostructures 1 or 2, the bacteria died.
This discovery was of great interest to us, because we believed we could use these findings to develop a new generation of bone implants with the ability to kill bacteria—without the need to use antibiotics [1]. We hope that implants with bacteria-killing nanostructures on them will prevent infections and improve the quality of life of patients who need implants to repair their bone defects.
Titanium—A Good Material for Bone Implants
The first difficulty scientists face when developing implants for bone repair is the biocompatibility of the material, which means that this material must not be toxic to the host or cause immunological rejection. Since the materials used to create implants are foreign to the body, the body could consider the implant a threat and attempt to reject it. Therefore, scientists must look for materials that do not trigger harmful processes in the body but that do remain stable in the body over time. To accomplish this, the pillar-like nanostructures on cicada wings were created on titanium surfaces. Titanium is a well-known metal often used in medicine because it has mechanical properties close to those of the bone, it remains stable over time, and the body is unlikely to react to it. Therefore, titanium appears to be a safe material for medical implants that are in close contact with various body tissues over a long period of time.
Testing the Material
When developing devices for medical purposes, it is mandatory to put the devices through various types of testing. Testing can tell us whether the device is toxic to the body and can give us a preliminary idea of how effective the device might be. To get this information, we must conduct what is called an in vitro study. This means that we perform laboratory experiments using cells or microorganisms. By studying the behavior of cells, we can get a partial picture of what might happen in an organism. Humans have many types of cells, each with their own jobs. We needed to test our devices using cells that would tell us the most about bone regeneration, because that is what we wanted our titanium implants to help with. One cell type is of great interest for bone repair—mesenchymal stem cells (MSCs). MSCs are amazing in that they can develop into many different types of cells depending on their environment. When MSCs are present at the site of a bone defect, they normally pick up signals from the environment and become osteoblasts—the bone-forming cells. So, we used MSCs in our experiments to mimic what would occur on our titanium implant once it was inside the body. We grew MSCs on our implants for several days.
What Did We Find?
Remember that the titanium we are studying kills bacteria thanks to the nanostructures on its surface. Therefore, we wondered if our nanostructures would also be harmful to human cells. Fortunately, this was not the case! MSCs survived and grew normally on the surfaces of our engineered titanium (Figure 3). What was even more surprising was that the MSCs on our nanostructured titanium started to become osteoblasts. A cocktail of molecules in the MSC environment usually controls the transition from one cell type to another, but we did not use any known molecule to trigger this switch in our experiments. We deduced that the nanostructures were probably perceived as a signal for the MSCs to become osteoblasts [3]. This could be another great feature of our nanostructured titanium implants.
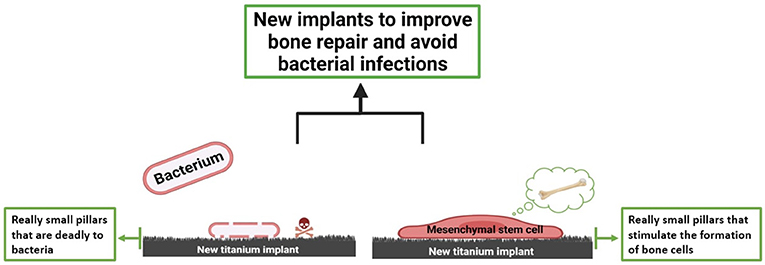
- Figure 3 - Our engineered titanium surfaces have two functions.
- First, they can cause bacterial death. Secondly, they can stimulate MSCs to become osteoblasts once the MSCs adhere to the nanostructures. This opens the possibility of creating new bone implants that would help bone reconstruction, while limiting the bacterial infections that are common in this type of surgery (Figure created with BioRender.com).
Conclusion
Our nanostructured titanium has incredible potential. Without adding antibiotics, the material itself can kill bacteria. Additionally, the nanostructures can help the body to create new bone. So, by mimicking structures that exist in nature, we developed a new type of material that could be extremely useful in medicine. Thanks to our preliminary exciting results, we are working to extend the study to other cell types involved in bone regeneration. This will help us to understand how nanostructures affect the behavior of those cells. Ultimately, we hope to prevent bacterial infections on implants and to create implants that are well-accepted by the human body. This would be a major step toward safe, effective bone-repair surgery that would improve the lives of many patients while simultaneously reducing the need for antibiotics.
Glossary
Osteoblasts: ↑ Bone-producing cells.
Bone Regeneration: ↑ Natural process of bone formation, which is involved during fracture healing.
Antibiotics: ↑ Medicines to treat infections by killing bacteria.
Nanostructures: ↑ Small structures, at the nanometer scale, on top of a surface. A nanometer is one billionth of a meter.
Biocompatibility: ↑ To be biocompatible, that means compatible with a living organism, a material must not be toxic to the host or cause immune reactions.
In vitro: ↑ This term refers to experiments that are done in laboratory out of a living organism.
Mesenchymal Stem Cells (MSCs): ↑ Immature cells that can become many different cell types, including fat cells, bone cells, or cartilage cells, depending on signals from the environment.
Acknowledgments
We thank the Gueules Cassées Fundation for their support.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Original Source Article
↑Le Clainche, T., Linklater, D., Wong, S., Le, P., Juodkazis, S., Le Guével, X., et al. 2020. Mechano-bactericidal titanium surfaces for bone tissue engineering. ACS Appl. Mater. Interfaces. 12:48272–83. doi: 10.1021/acsami.0c11502
References
[1] ↑ Linklater, D. P., Baulin, V. A., Juodkazis, S., Crawford, R. J., Stoodley, P., and Ivanova, E. P. 2021. Mechano-bactericidal actions of nanostructured surfaces. Nat. Rev. Microbiol. 19:8–22. doi: 10.1038/s41579-020-0414-z
[2] ↑ Le Clainche, T., Linklater, D., Wong, S., Le, P., Juodkazis, S., Le Guével, X., et al. 2020. Mechano-bactericidal titanium surfaces for bone tissue engineering. ACS Appl. Mater. Interfaces. 12:48272–83. doi: 10.1021/acsami.0c11502
[3] ↑ Halim, A., Ariyanti, A. D., Luo, Q., and Song, G. 2020. Recent progress in engineering mesenchymal stem cell differentiation. Stem Cell Rev. Rep. 16:661–74. doi: 10.1007/s12015-020-09979-4