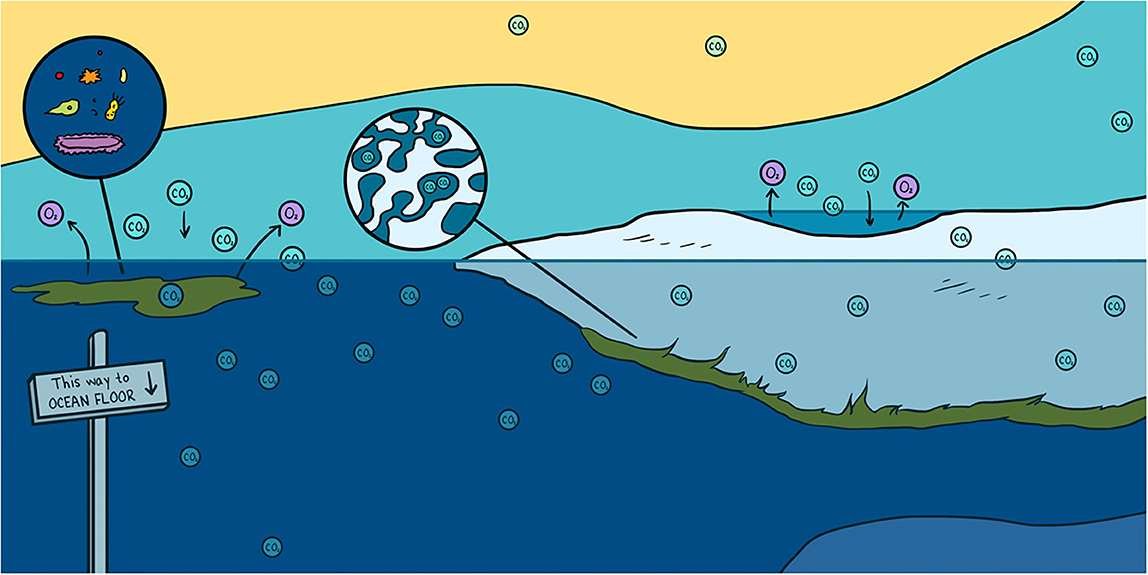
Abstract
Every winter, a frozen blanket known as sea ice completely covers the Arctic Ocean. For centuries, sea ice has been viewed as a solid lid on the ocean that acts as a boundary to block gases traveling between the ocean and the atmosphere. However, scientific discoveries over recent years have shown that sea ice is more like a sponge, a porous substance that is also home to microscopic life forms. The pores in sea ice are filled with very salty liquid called brine that is rich in carbon dioxide (CO2). These liquid pockets create a network of tubes or channels that move gases like CO2, similar to the way veins and arteries move blood in our bodies. In this article, you will discover how CO2 enters, exits, and is transformed in one of the harshest environments on Earth.
Sea Ice: A Zoomed-out View
On average, sea ice covers about 23 million square kilometers of the Earth’s oceans, or about two-and-a-half times the area of Canada [1]. Due to its size, sea ice is visible from space as a large white blanket on the ocean. By observing sea ice at these vast scales, we can see dramatic changes to the ice extent throughout the year and over decades—since 1978, when the first satellite observations of sea ice were made.
Each year, as the sun sets and winter begins in the Arctic Ocean (in the far North) or the Southern Ocean (in the far South of Antarctica), sea ice forms when air temperatures decrease, and the ocean begins to freeze. As winter continues, sea ice thickens and grows outwards to cover vast areas of the ocean. In some places in the Arctic, sea ice even grows to be many meters thick! As the sun rises and the air warms in the spring, the sea ice begins to melt and break up, exposing the liquid ocean below. We call this expansion and contraction of sea ice a seasonal cycle (Figure 1).
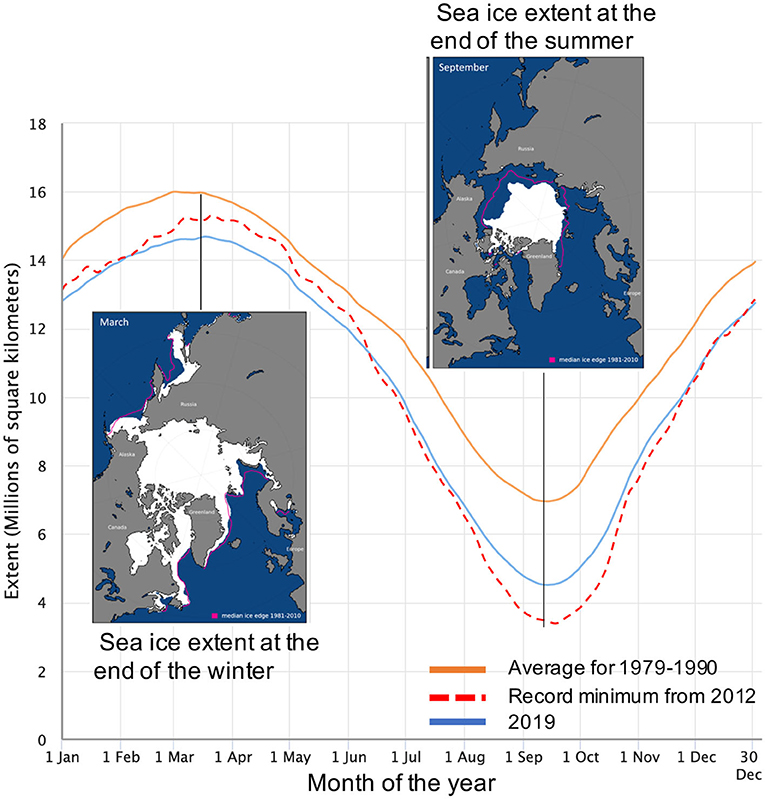
- Figure 1 - The seasonal sea-ice cycle in the Arctic.
- The orange line shows the monthly historical average from 1979 to 1990. The red line shows 2012, when the ice reached a record minimum. 2019 is shown in blue. The left insert shows the sea ice cover at the end of the winter 2019 and the right insert shows it at the end of the summer. Since 2000, the summer sea ice extent has drastically decreased. Maps and date are from the National Snow and Ice Data Center (NSIDC), University of Colorado, Boulder, CO.
Comparing sea ice from year to year, we find that the amount of sea ice covering the ocean is changing. This long-term change is happening as the sea ice continues to grow and melt as part of its yearly seasonal cycle (Figure 1, blue and orange lines). In the Arctic, sea ice is melting more in summer than it used to, and we have already lost 30% of the summer sea ice since 1990 (Figure 2, yellow line). Scientists predict that, by 2050, all the Arctic sea ice will completely melt during summer for the first time in history. This means that, although explorers can walk to the North Pole today, in the future they will have to sail to it. One of the great research questions of our time is how these changes are affecting ocean life and our warming climate.
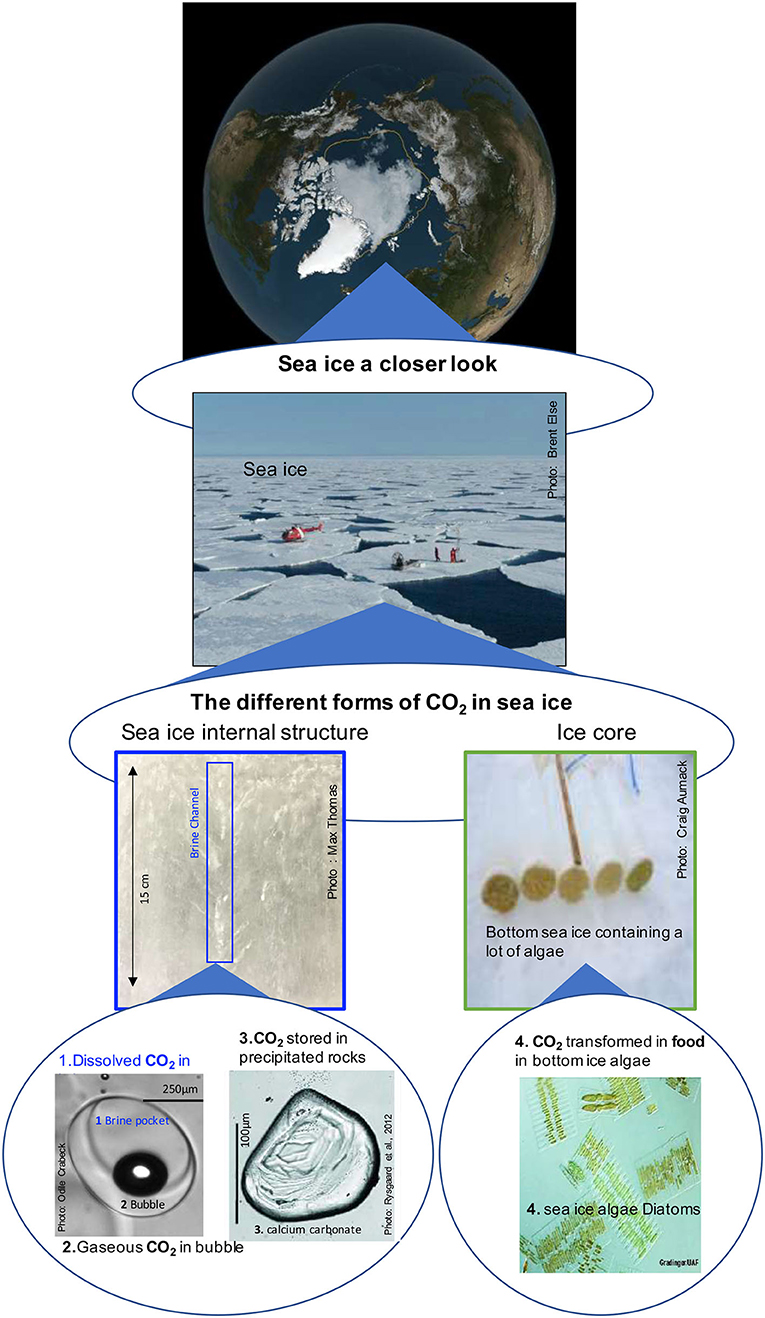
- Figure 2 - Sea ice a closer look.
- On the top panel, a satellite view of the Arctic Ocean at the end of summer 2019. The yellow line shows the historical (1979 to 1990) extent of the sea ice summer cover. We can observe that there is now less ice in the Arctic during the summer. Before the summer sea ice cover was reaching beyond the yellow line. The middle panel shows scientists sampling sea ice during the summer season. The bottom panel shows on the left-side the sea ice internal structure and on the right-side, ice cores that contain a lot of algae at the bottom. This bottom panel shows also, where the CO2 is trapped in sea ice:
- In brine where the CO2 is stored in a dissolved state,
- In bubbles where the CO2 is stored in gas phase,
- In crystals of calcium carbonate where the CO2 is stored in solid form as rocks,
- In sea ice algae where the CO2 is stored as carbon (sugars food) (http://www.arcodiv.org/seaice/diatoms/IceDiatoms.html).
Sea Ice: A Closer Look
Zooming in on sea ice, to the scale of only a few centimeters, shows that it is complex. Pockets of salty liquid, known as brine, exist in the sea ice (Figure 2). Brine pockets are liquid at temperatures below zero because the salt prevents the liquid from freezing, and there is always some liquid in sea ice [2]. Zooming in still further we find gas bubbles, salt crystals, and life within these brine pockets (Figure 2, bottom panel). These brine pockets are a unique habitat for microscopic organisms and a place where chemical reactions happen. Scientists have been working to understand sea ice at these very small scales and see how sea ice affects the chemical nature of the oceans and even life beyond the oceans.
CO2 in the Atmosphere, Oceans, and Living Organisms
Carbon is one of the most abundant elements on Earth, along with oxygen, nitrogen, and hydrogen. Carbon is found in the atmosphere as carbon dioxide (CO2) gas, in the ocean as dissolved CO2, in some kinds of rock, and in all living organisms. Carbon is essential to life and you are made of about 20% carbon.
In the atmosphere, CO2 is a major gas that contributes to global warming [3]. CO2 emitted by human activities (cars, the oil/gas industry, etc.) can move between the atmosphere, the oceans, and living organisms, and it changes forms as it moves. If CO2 is pumped into the deep ocean, it can be locked up there for hundreds of years, reducing global warming. The processes that move CO2 from the atmosphere into the ocean are called pumps. There are two main CO2 pumps in the ocean: the solubility pump and the biological pump. Oceans have already absorbed one-third of the CO2 emitted by human activities thanks to the solubility and biological pump.
The solubility pump (Figure 3, blue arrows) refers to the process by which atmospheric CO2 is absorbed by the ocean surface and become dissolved in the surface ocean. Once the CO2 is dissolved, it can be transported deep into the ocean by the ocean currents. The capacity of the ocean to take up CO2 from the atmosphere depends on the water temperature and salinity (saltiness). Cold, freshwater can absorb more CO2 than warm, salty water. Therefore, the cold polar oceans, like the Arctic Ocean, are great at taking up CO2 from the atmosphere. If the CO2 goes to the bottom of the ocean, it can stay there for 1000 years or more (Figure 3).
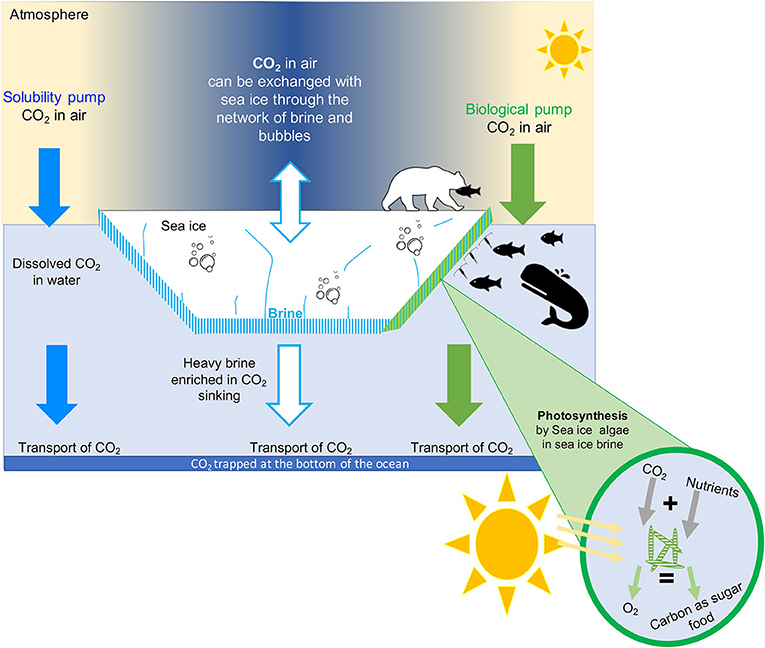
- Figure 3 - How does CO2 move from the atmosphere into the ocean?
- CO2 is found in the atmosphere as a gas. This CO2 can dissolve in the seawater. Once in the seawater, it can be transported by currents to the bottom of the ocean. We call this the solubility pump (blue arrow). Sea ice can also exchange CO2 with the atmosphere. The CO2 in sea ice is either dissolved in brine or stored as gas in bubble or in solid phase as calcium carbonate rocks. Some of the brine containing CO2 is rejected to the underlying seawater and transported to the interior part of the of the ocean. This process trapped CO2 at the bottom of the ocean for a very long time (white arrow). Sea-ice algae also use CO2 to grow, through the process of photosynthesis. We call this the biological pump (green arrow). The CO2 stores in algae as food can move up the food chain as the algae is eaten by grazers, which are then eaten successively by larger animals.
The biological pump refers to the use of CO2 by algae called phytoplankton. Algae are microscopic, single-celled organisms that grow using sunlight, nutrients, and CO2 in the chemical process of photosynthesis [4] (Figure 3, green arrows). Many phytoplankton live floating in the world’s oceans. Because algae use CO2 to grow, they help the ocean to take up CO2 from the atmosphere (Figure 3, green arrows). Some of these algae are specialized to live in the unique environment provided by sea ice, including in the liquid brines.
How Does Sea Ice Algae Transform CO2?
I am only as big as a cell. I breathe in CO2 and breath-out O2. I live in salty brine pocket, and I get my energy from the sunlight. Who am I? I am a sea ice algae.
Ice algae grow in brine pockets within the ice, in meltwater ponds at the surface, and most importantly at the base of sea ice, where the ice is touching the ocean below. Ice algae grow quickly, or bloom, when light becomes available for photosynthesis in the spring (Figure 3, green arrows). Ice algae known as diatoms are very good at growing quickly and largely make-up the ice-algae bloom. Although the diatoms are too small to count individually, scientists can see the bloom as a browning of the ice (Figure 2, bottom panel). The ice-algae bloom lasts until late summer when the cells have used up the nutrients needed to grow and when the ice around them begins to melt. Photosynthesis performed by ice algae can have a large impact on how much CO2 sea ice takes up in the spring (Figure 3, green arrows). Generally, when the ice is brown with algae, it is expected that lots of CO2 is being taken up into the ice (Figure 2, bottom panel). Ice algae play an important role in using atmospheric CO2, but they are also important for the animals living in the Arctic Ocean. The growth of ice algae supplies other organisms with a lot of food. The size of the algae bloom means that organisms can get lots of food very easily, like going to a vegetarian buffet for dinner. Nutrition gained from ice algae is transferred up the food web as one organism eats another, all the way to the polar bear.
What Happens to CO2 Trapped in Sea Ice?
As sea ice forms in winter, it traps salts and CO2 from the ocean in brine (Figure 2, bottom panel). In fact, so much CO2 gets trapped with salt that it is transformed into solid rock by chemical reactions. One of the most common rocks that forms from CO2 inside sea ice is made of calcium carbonate, also called limestone, which is the same substance that makes up the skeletons of corals and many seashells you might find on the beach. Researchers can see the tiny pieces of calcium carbonate when they melt a core of sea ice and look at it under the microscope (Figure 2, bottom panel). The CO2 in sea ice is also trapped in bubbles (Figure 2, bottom panel). The bubbles can rise from the bottom of the sea ice to the surface through the brine channels. Once at the surface, the gases can escape into the air (Figure 3, white arrows). Sea ice also sends some CO2 to the bottom of the ocean. This process takes place during winter, when the salty brine from the sea ice sinks to the deep ocean, bringing CO2 along (Figure 3, white arrows). This is often referred to as the sea-ice pump, similar to the solubility and biological pumps described above. Through the rising bubbles and sinking brine, the sea ice loses a lot of CO2 that was trapped inside it. As a result, when the sun comes back in the spring, the sea ice no longer holds as much CO2. Researchers have observed that, in the spring, sea ice can again absorb lots of CO2 from the atmosphere. Overall, researchers think that sea ice helps the ocean to absorb CO2. So, sea ice helps us fight climate change.
Key Messages
Sea ice cover grows in winter and melts in summer. Thanks to the cold water and the presence of algae and sea ice, the Arctic Ocean is a carbon sink; it helps to decrease the amount of CO2 in the atmosphere. Firstly, sea ice algae use CO2 to grow and create food for larger organisms. Secondly, sea ice can trap CO2 in its brine and favor its transport to the bottom of the ocean. In the Arctic, the summer sea ice cover is strongly decreasing due to global warming. Global warming threatens the house of ice algae and the ability of the Arctic Ocean to exchange CO2 with the atmosphere.
Glossary
Sea Ice: ↑ Is frozen seawater that floats on the ocean surface. It is composed of ice crystal and salty liquid pocket called brine.
Brine: ↑ Is water with a high concentration of dissolved salt.
Solubility Pump: ↑ Is a process that takes up CO2 from the atmosphere to the ocean’s surface as dissolved CO2 and transports it to the bottom of the ocean.
Biological Pump: ↑ Contributes to the ocean’s role in taking up and storing CO2 from the atmosphere. The CO2 is transformed and stored by micro-organism as algae that use photosynthesis to grow.
Phytoplankton: ↑ Are single-celled algae that live at the surface of the ocean and use the photosynthesis process to grow. The most common types of phytoplankton are Diatoms. Phytoplankton form the base of aquatic food webs. They are used as food supply by small fish and other marines’ animals.
Photosynthesis: ↑ Is the process by which plants and algae make food. This chemical process uses sunlight, CO2, and nutrients to produce sugars that the cell can use as energy to grow.
Bloom: ↑ Is a rapid increase in the population of algae. An algal bloom is often recognized by the green or brown coloration of the water.
Calcium Carbonate: ↑ Is a sedimentary rock like limestone. Calcium carbonate is produced by the precipitation (solidification) of dissolved calcium and CO2 in water.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was a contribution to the Diatom-ARCTIC (Diatom Autecological Responses with Changes to Ice Cover) and EISPAC (Effects of ice stressors and pollutants on the Arctic marine cryosphere) project. This work was also supported by the international working group BEPSII: Biochemical exchange processes at Sea Ice Interfaces.
References
[1] ↑ Thomas, D. N. (Ed.). 2017. Sea Ice. Norwich:John Wiley & Sons.
[2] ↑ Glessmer, M. 2019. How does ice form in the sea? Front. Young Minds 7:79. doi: 10.3389/frym.2019.00079
[3] ↑ Hubbe, A., and Hubbe, M. 2019. Current climate change and the future of life on the planet. Front. Young Minds 7:37. doi: 10.3389/frym.2019.00037
[4] ↑ Ghosh, T., and Mishra, S. 2017. How does photosynthesis take place in our oceans? Front. Young Minds 5:34. doi: 10.3389/frym.2017.00034