Abstract
Neurons are cells contained within the brain and spinal cord that specialize in communicating information within the body. Neurons are important for many things including moving, breathing, thinking, and feeling pain. If these cells are injured due to an accident, for example, the body can no longer perform some of these important functions. As a result, a person can become disabled in some way. To help patients with injuries to their brains or spinal cords, scientists and doctors may be able to replace damaged neurons by transplanting new cells into the injured person. By using new cells to replace the neurons lost from injury, it is possible that patients will recover some of their lost abilities, such as moving. Scientists think that stem cells are the ideal cell type to transplant into injured patients, because stem cells can multiply and change into the different cell types needed to repair the injury. The stem cells that researchers transplant can be made in the lab from skin cells and blood cells. Skin and blood cells can both be obtained using a needle. Currently, stem cells from patients with brain disease, like Alzheimer’s disease, are used to study these diseases in the laboratory so that cell replacement therapies can be developed.
Introduction
In this article, you will learn about what neurons (new-rons) are and how scientists and doctors hope that they may be used to help repair the central nervous system. The central nervous system is made up of the brain and spinal cord (Figure 1). Neurons are the special cells contained within these structures. The brain and the spinal cord are very important for all sorts of things in the body—like moving, breathing, thinking, and feeling pain—and, when the brain or spinal cord is injured, this can cause serious problems for the person. This article will explain how neurons can be used to help repair these injuries, and it will also teach you about stem cells and how some kinds of stem cells can be altered in the laboratory to help them to become neurons, which may then be used to help people with injuries in their central nervous systems. Finally, the article will discuss how neurons made from stem cells are already being used and how doctors and scientists hope they might be used in the future.
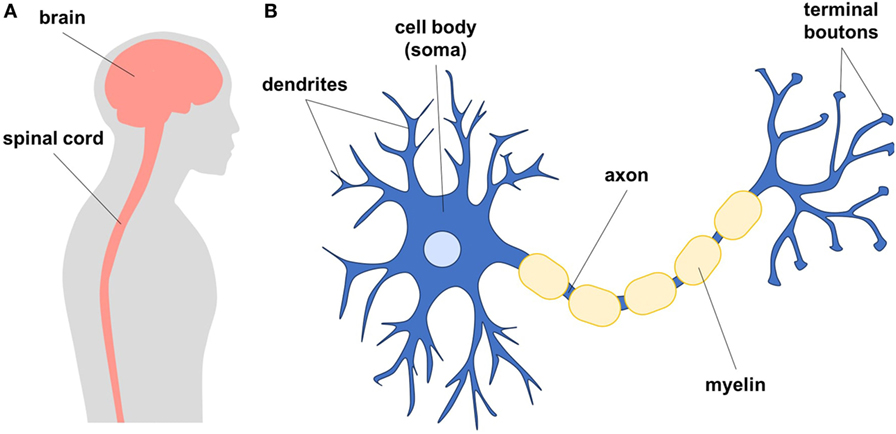
- Figure 1
- A. Diagram showing the human brain and spinal cord, which together are called the central nervous system. The central nervous system is made up of neurons and glial cells. B. Neurons are cells that form networks to communicate. Neurons have a cell body (soma), tree-like structures called dendrites, and long axons that end in terminal boutons to send signals to other cells. Glial cells are star-shaped cells that help maintain neurons in a healthy environment. The axons of neurons are wrapped in protective insulation (myelin) to make the communication between cells faster.
What are Neurons?
Neurons are the main communicating cell type of the nervous system. Each neuron has a cell body, which keeps the cell alive by making proteins and energy (Figure 1). The cell body also acts as the brain of the cell, because it processes all the incoming information and tells the neuron what to do. From the cell body, long axons (axe-ons) and dendrites branch out to send and receive messages to and from other cells. At the end of the axon, there are structures called terminal boutons, which look like small bumps. The terminal boutons contact dendrites of other neurons to form specialized connections called synapses (sin-ap-sez). When neurons talk to each other, they send electrical or chemical messages that can cause the next neuron to fire an action potential (signal) down its axon. In this way, the signal can be sent along a whole chain of neurons. To speed up the signaling, many neurons are insulated by fatty layers called myelin, similar to the way a wire is insulated. Within your brain, your neurons help you learn to read, write, ride a bicycle, form memories, and even to have emotions. Neurons are the building blocks that make us who we are and that allow the many complex processes of the brain to occur. Neuronal signals can also travel long distances from your brain through the spinal cord to tell your muscles to move your arms and legs. There are also neurons that can bring touch information from your skin back to your brain.
How Can Neurons be Used to Treat Nervous System Diseases?
In many nervous system diseases, neurons are damaged and die. Scientists call this process neurodegeneration (new-ro-dee-gen-ner-ay-shon). The biggest neurodegenerative diseases are Alzheimer’s disease and Parkinson’s disease. In Alzheimer’s disease, neurodegeneration causes people to forget things. In Parkinson’s disease, neurons in a part of the brain responsible for movement are lost, and this makes it difficult for these patients to walk and move. If someone has a bad fall or gets into a car accident, neurons can also be injured. If the head is affected, we call this traumatic brain injury. If the spinal cord is affected, we call this traumatic spinal cord injury. The loss of neurons breaks the chain of messages traveling through the nervous system, which is required for normal function.
One possible way to treat neurodegenerative diseases and injuries of the central nervous system is to replace dead or damaged neurons through cell transplants. If new neurons can replace the lost ones, patients might be able to regain functions such as memory or movement; however, there are some important challenges to overcome. First, there is no easy way to collect neurons from the body to use as a treatment. There are a limited number of neurons in the brain and spinal cord, which are both difficult areas to access, and neurons mostly do not grow back. Second, neurons are not the only cells affected by neurodegeneration. There are other cells in the nervous system known as glial cells, which surround neurons and provide support, protection, and supply nutrients to the neurons. Glial cells can be lost through diseases and injury, too.
What are Pluripotent Stem Cells?
To overcome these obstacles, researchers have looked at a special kind of cell called stem cells, which can do two important things. Stem cells can self-renew, meaning they can continuously make new copies of themselves; and stem cells can differentiate (dif-ur-en-shee-ate), meaning they can transform into other types of cells. The first studied stem cell was the embryonic stem cell (ESC). ESCs are pluripotent, meaning they can differentiate into any type of cell in the body, from heart cells to brain cells to muscle cells (Figure 2). However, ESCs cannot be made from your own cells—they must come from embryos, and so the source of cells is limited [1]. More recently, scientists discovered that stem cells with properties similar to ESCs could be made from any cell in the body, by adding specific molecules that send signals into the cells. These cells are called induced pluripotent stem cells (iPSCs) [2]. iPSCs are useful because they have all of the functions of ESCs, but they can be made from a person’s own cells [3]. Furthermore, the iPSCs can be made from skin cells, which are easy to obtain without the need for surgery, and there are plenty of skin cells available.
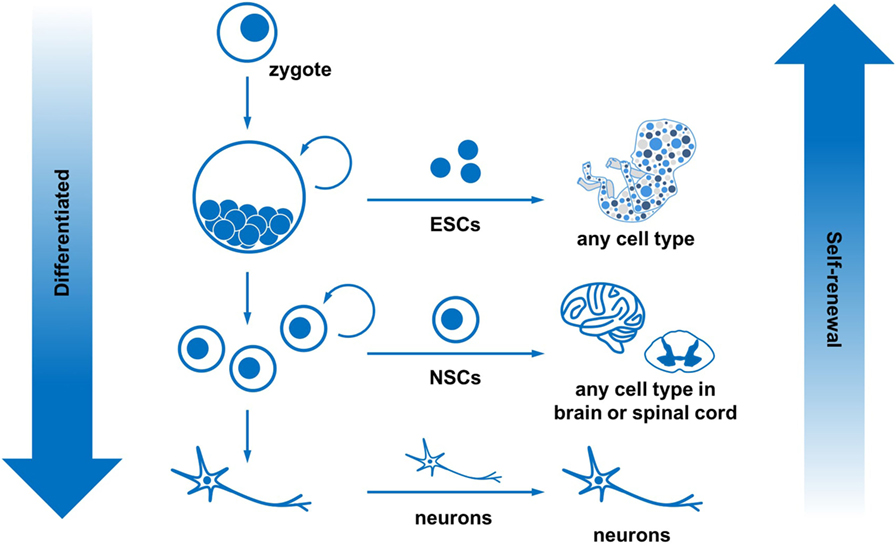
- Figure 2
- Stem cells can self-renew and differentiate into many cell types. All the cells in the body come from the zygote. The zygote continues to divide into embryonic stem cells, which can form any cell in the body. As these stem cells continue to divide and grow, they become more specialized. For example, neural stem cells can only become cells within the brain and spinal cord. Eventually, cells become very specialized, like neurons, and can no longer self-renew. In summary, as cells become more differentiated, they have a reduced ability to self-renew.
What are Neural Stem Cells (NSCS)?
Neural stem cells are normally found within the brain and spinal cord. NSCs are specialized stem cells that can differentiate into the cell types of the central nervous system, including neurons and glial cells (Figure 2). Scientists have now found ways to use ESCs and iPSCs to make NSCs, again by using molecules that send specific signals into the cells (Figure 3). Exciting research in the last few years has also uncovered ways to make NSCs directly from any other cell in the body without going through the iPSC stage—these are called directly reprogrammed NSCs. Directly reprogrammed NSCs can be made using chemical mixtures or by editing of the cell’s DNA. This is very exciting, because it means we have a nearly limitless supply of NSCs, which can become the neurons and glial cells needed to repair the central nervous system.
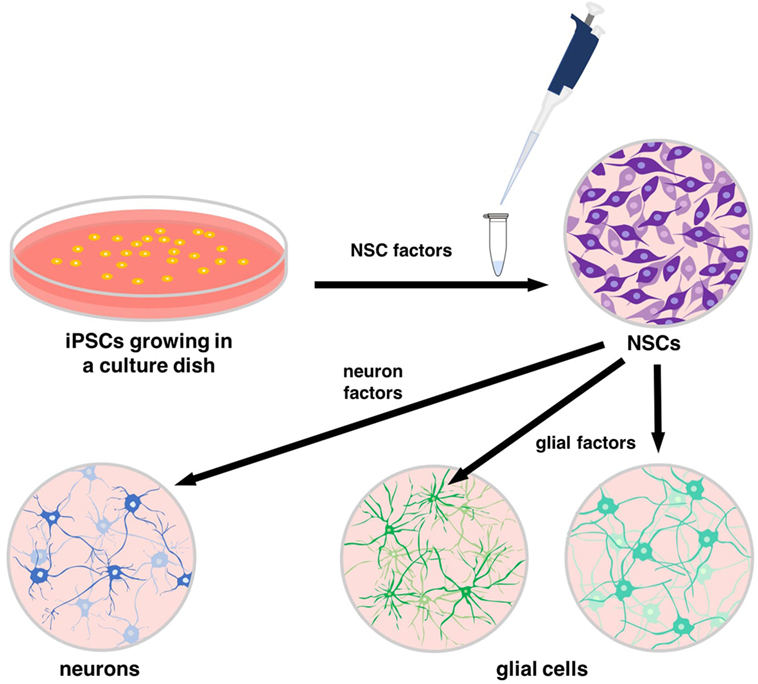
- Figure 3
- Specific proteins, known as neural stem cell (NSC) factors, can be added to induced pluripotent stem cells (iPSCs) to turn them into NSCs. NSC factors interact with the DNA and proteins of iPSCs to provide the correct signals for differentiation. Additional sets of proteins can signal for NSCs to become cells of the central nervous system, including neurons and glial cells.
How Do Scientists Make iPSCS, NSCs, and Neurons Today?
All cells in the body are a product of cell divisions that started from the very first cell, which is called a zygote (Figure 2). The zygote divides and then each of the new cells divides, and this happens continuously until all the cells of the body have formed. The cells in the body are a mix of stem cells and specialized cells that have differentiated. Within the central nervous system, pluripotent stem cells turn into NSCs and then to neurons and glial cells. This complex pathway is guided by a series of very specific proteins, which bind to regions of DNA and as a result influence the function of other proteins [4]. All of these interactions result in changes to the identity of the cells. To generate the correct types of central nervous system neurons, these proteins should be expressed in a specific pattern and combination. To make neurons in vitro (in the lab, within a culture dish), we need to copy all of the steps and timing that occur in vivo (in the body). To do this, scientists make iPSCs from a patient’s cells (most often skin or blood cells) [2]. Then, these cells are differentiated in vitro into NSCs, using the same signaling proteins and molecules that are found in vivo. After this, the NSCs are purified and allowed to divide until there are large numbers of them. Then, new signaling molecules and proteins are added, again mimicking what happens in the body, to differentiate the cells into neurons and glial cells. The entire process requires several months of carefully planned steps. At each step, the identity of the cells is checked using various techniques, to make sure the differentiation process is proceeding as expected. The final cell product can be used in the lab to study nervous system injuries or as part of clinical trials (careful monitored experiments in patient volunteers).
How are Neurons Made from Stem Cells Being Used?
Induced pluripotent stem cells are a useful cell source, because they can be made from the cells of the same person they will be used to treat (these are called autogenic iPSCs). Because this field of study is so new, the first clinical trials using autogenic iPSCs are still in the planning stages. However, we can still use iPSCs cells to study several important things in the lab. For example, iPSCs and the neurons made from them are used to study neurodegenerative diseases like Alzheimer’s disease, Parkinson’s disease, traumatic injury, and depression in vitro. When cells are used in the lab to study a disease that normally occurs in the body, this is called a model of the disease. These types of cell models are important, because they can be made from human cells, which are easier to get and cheaper to use than cells from animals. In fact, a recent study used iPSCs from patients with Alzheimer’s disease and made them into a three-dimensional cell model [5]. The researchers showed that there is a buildup of abnormal protein in the cell model that is just like what happens in the in vivo disease. So, using the model, different treatment drugs can then be tested on these abnormal cells to identify the best possible treatments.
Neurons from stem cells are also being developed into cell replacement therapies to be tested in animal models of disease. For example, scientists are transplanting NSCs made from iPSCs into rats to repair the spinal cord after injury (Figure 4). This has allowed scientists to uncover and solve some important problems before the stem cells are used in clinical trials in humans. The animal models have also allowed scientists to combine different treatments with stem cells to achieve the best possible outcomes [6]. Some of the problems that scientists are now working on include improving the survival of stem cells after transplantation and figuring out ways to encourage the transplanted cells to make connections with the patient’s neurons [7].
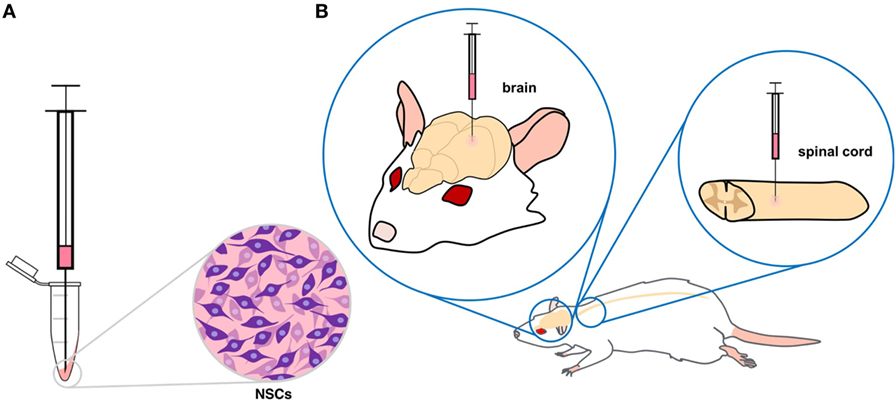
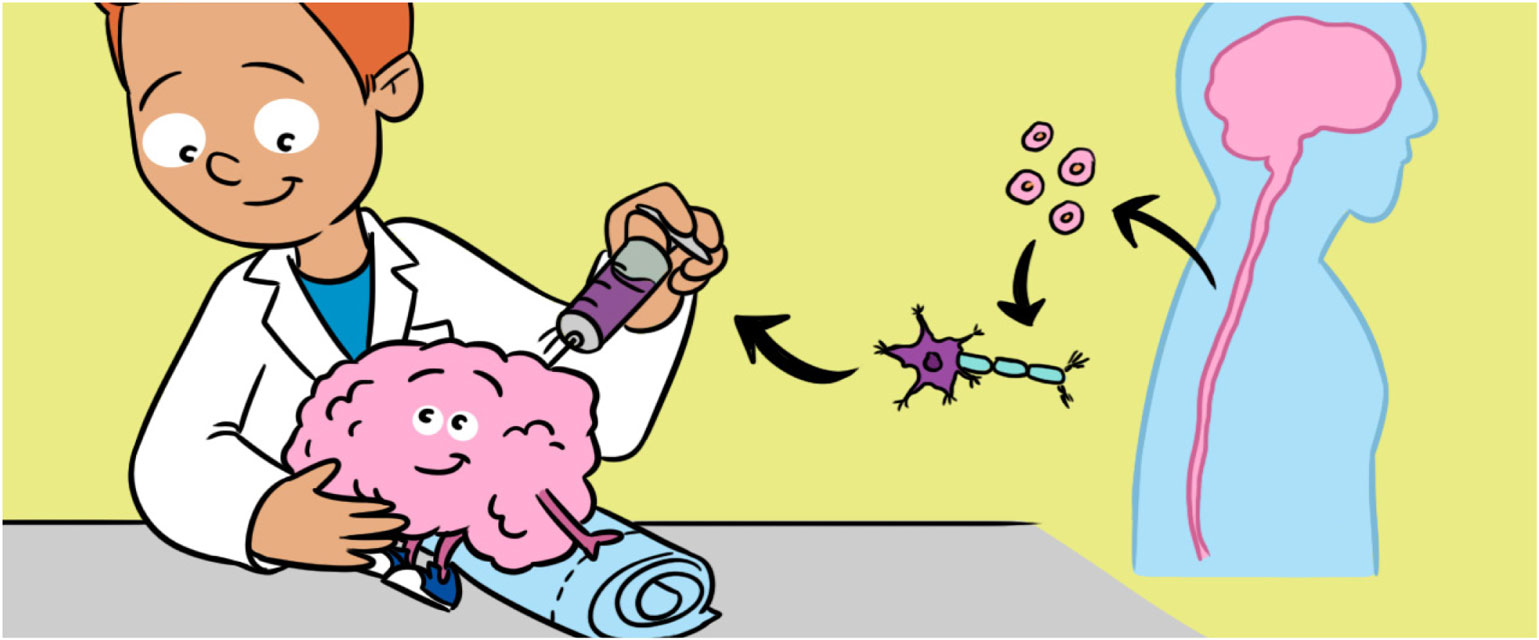
- Figure 4
- A. Neural stem cells (NSCs) that have been collected in a tube are drawn into a syringe. B. Depending on where the damaged neurons are found, NSCs can be transplanted into either the brain or spinal cord.
How Might Neurons Made from Stem Cells be Used in the Future?
Since the discovery of methods to make ESCs and iPSCs into NSCs and neurons, there has been a lot of excitement about how this can be used to treat diseases of the brain and spinal cord. Over the next 10–20 years, we expect that scientists will figure out how to make NSCs and neurons survive better after they have been transplanted into patients. We also expect that new methods will be found for making connections between the transplanted cells and the neurons of the person or animal being treated. These developments may allow us to use stem cells to successfully treat diseases like Parkinson’s disease, Alzheimer’s disease, multiple sclerosis, traumatic brain injury, stroke, spinal cord injury, and a long list of other problems that affect people. However, the goal of successfully treating these diseases cannot be achieved by a single person. Finding stem cell-based treatments for all of these different problems will require teamwork from people around the world, including scientists, doctors, governments that fund research, patients that are willing to participate in clinical trials, and companies that will help grow large numbers of stem cells. This effort could also use the help of the next generation of young scientists, who can bring new ideas to the field and help push this research forward.
Key Summary Points
- Neurons are the main communicating cell type of the nervous system
- When neurons die or get damaged, some of them can be replaced through cell transplants
- Researchers prefer transplanting stem cells rather than neurons, because stem cells can self-renew and they can differentiate into different cell types
- iPSCs and NSCs can be made from skin cells and blood cells, using a series of special proteins as signaling molecules
- Researchers are using stem cells to model neurodegenerative diseases and to develop cell replacement therapies
Glossary
Neurons: ↑ The main communicating cell type of the central nervous system.
Central: ↑ The organ system that controls all our thoughts and actions; the brain and spinal cord.
Stem: ↑ Specialized cells that can multiply and change into the different cell types.
Glial: ↑ Cells of the central nervous system that surround neurons to provide support, protection, and nutrients.
Self: ↑ To continuously be able to make new copies of oneself.
Differentiate: ↑ To transform into other cell types.
Pluripotent: ↑ The ability to differentiate into any cell type in the body.
Induced: ↑ Stem cells that are made from any cell type in the body by adding specific signaling molecules.
Neural: ↑ Stem cells that can self-renew but can only differentiate into cells of the central nervous system.
In Vitro: ↑ In the lab, in a culture dish.
In Vivo: ↑ In the body.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
[1] ↑ Mothe, A. J., and Tator, C. H. 2013. Review of transplantation of neural stem/progenitor cells for spinal cord injury. Int. J. Dev. Neurosci. 31:701–13. doi:10.1016/j.ijdevneu.2013.07.004
[2] ↑ Takahashi, K., Tanabe, K., Ohnuki, M., Narita, M., Ichisaka, T., Tomoda, K., et al. 2007. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131:861–72. doi:10.1016/j.cell.2007.11.019
[3] ↑ Khazaei, M., Ahuja, C. S., and Fehlings, M. G. 2016. Induced pluripotent stem cells for traumatic spinal cord injury. Front. Cell. Dev. Biol. 4:152. doi:10.3389/fcell.2016.00152
[4] ↑ Sadler, T. W. 2005. Embryology of neural tube development. Am. J. Med. Genet. C Semin. Med. Genet. 135C:2–8. doi:10.1002/ajmg.c.30049
[5] ↑ Lee, H. K., Velazquez Sanchez, C., Chen, M., Morin, P. J., Wells, J. M., Hanlon, E. B., et al. 2016. Three dimensional human neuro-spheroid model of Alzheimer’s disease based on differentiated induced pluripotent stem cells. PLoS One 11:e0163072. doi:10.1371/journal.pone.0163072
[6] ↑ Zweckberger, K., Ahuja, C. S., Liu, Y., Wang, J., and Fehlings, M. G. 2016. Self-assembling peptides optimize the post-traumatic milieu and synergistically enhance the effects of neural stem cell therapy after cervical spinal cord injury. Acta Biomater. 42:77–89. doi:10.1016/j.actbio.2016.06.016
[7] ↑ Ahuja, C. S., Wilson, J. R., Nori, S., Kotter, M. R. N., Druschel, C., Curt, A., et al. 2017. Traumatic spinal cord injury. Nat. Rev. Dis. Primers 3:17018. doi:10.1038/nrdp.2017.18